Journal of Modern Foreign Psychology
2024. Vol. 13, no. 1, 78–91
doi:10.17759/jmfp.2024130107
ISSN: 2304-4977 (online)
Experimental Design and Behavioral Testing Protocol for the Evaluation of Cognitive Abilities and Social Behavior in Mice Following Early Life Stress
Abstract
This manuscript presents a protocol designed for the comprehensive investigation of early life stress (ELS) outcomes and a feasibility study conducted with this protocol. ELS alters normal development by interfering at various levels: hormonal changes, brain cellular architecture, epigenome, and chromosomal structural elements. The protocol combines classic behavioral tests with advanced molecular techniques to obtain comprehensive data and thus uncover the underlying mechanisms of ELS. In this protocol, the main source of stress is maternal separation. Briefly, a group of C57Bl/6 mice undergoes maternal separation; then, mice perform the radial maze test and the resident-intruder test. As a control, another group of mice stays undisturbed and performs the same behavioral tests in the same timeframe. After the behavioral tests, biosamples are collected, including urine for corticosterone measurements, peripheral blood, hippocampus, amygdala, and prefrontal cortex tissues for DNA isolation and its downstream analyses (DNA methylation profiling and telomere length measuring), and whole brains for immunohistochemistry analysis of the glucocorticoid receptor density. This protocol was successfully tested as a feasibility study for a large-scale investigation that addresses potential flaws to establish a robust methodology. This paper reports on a comprehensive approach to examining multiple aspects of development that interrogates a holistic analysis of multilayer and multidimensional data and may contribute valuable insights for both animal and human studies.
General Information
Keywords: early life stress, working memory, animal models, HPA axis
Journal rubric: Neurosciences and Cognitive Studies
Article type: scientific article
DOI: https://doi.org/10.17759/jmfp.2024130107
Funding. This research was supported by the award from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) to the University of Houston and the Texas Center for Learning Disabilities (P50HD052117, PI: Jack Fletcher).
Acknowledgements. The authors are grateful to Drs. Olga Burenkova (Mason Laboratory, Department of Integrative Biology, University of Guelph), Fatin Atrooz, and Samina Salim (College of Pharmacy, University of Houston) for their assistance in designing, preparing, and executing the described experiments, and Aidan Nichols, Noelle Nguyen, Harini Kanamarlapudi, and Wu Wen-Wen (undergraduate students, University of Houston) for their assistance in performing the maternal separation procedure.
Received: 31.01.2024
Accepted:
For citation: Khafizova G.V., Naumova O.Y., Lopez A.L., Grigorenko E.L. Experimental Design and Behavioral Testing Protocol for the Evaluation of Cognitive Abilities and Social Behavior in Mice Following Early Life Stress [Elektronnyi resurs]. Sovremennaia zarubezhnaia psikhologiia = Journal of Modern Foreign Psychology, 2024. Vol. 13, no. 1, pp. 78–91. DOI: 10.17759/jmfp.2024130107.
Full text
Introduction
Materials and Methods
Animals
Maternal separation
Behavioral assessments
Radial arm maze test
Social defeat paradigm
Urine collecting for corticosterone measurement
Blood collecting for methylation profiling
Brain sectioning for methylation profiling and telomere length analysis
Brain dissection for immunohistochemistry (IHC)
Results
|
PND |
Females (grams; ± st.dev) |
Males (grams; ± st.dev) |
||
|
|
Control nest |
Jackson Lab |
MS nest |
Jackson Lab |
|
31 |
12.5 ± 1.29 |
14.7 ± 1.8 |
11.7 ± 3.5 |
16.5 ± 2.6 |
|
35 |
13.5 ± 2.1 |
17.8 ± 1.1 |
13.3 ± 2.5 |
20.7 ± 1.8 |
|
47 |
17 ± 0.58 |
18.75 ± 0.95 |
17.7 ± 2.1 |
22.75 ± 1.65 |
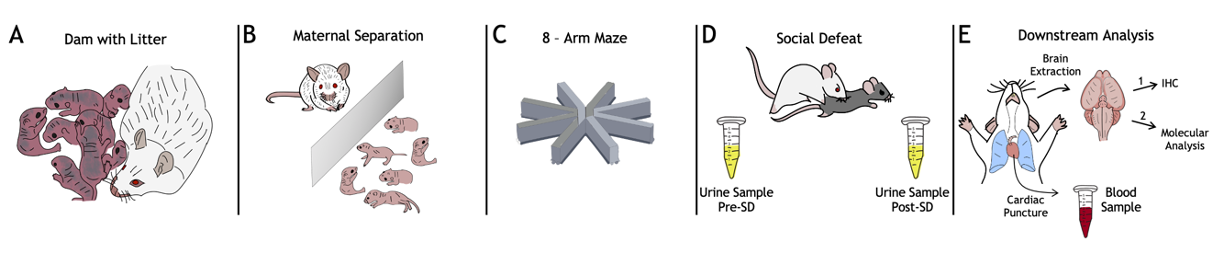
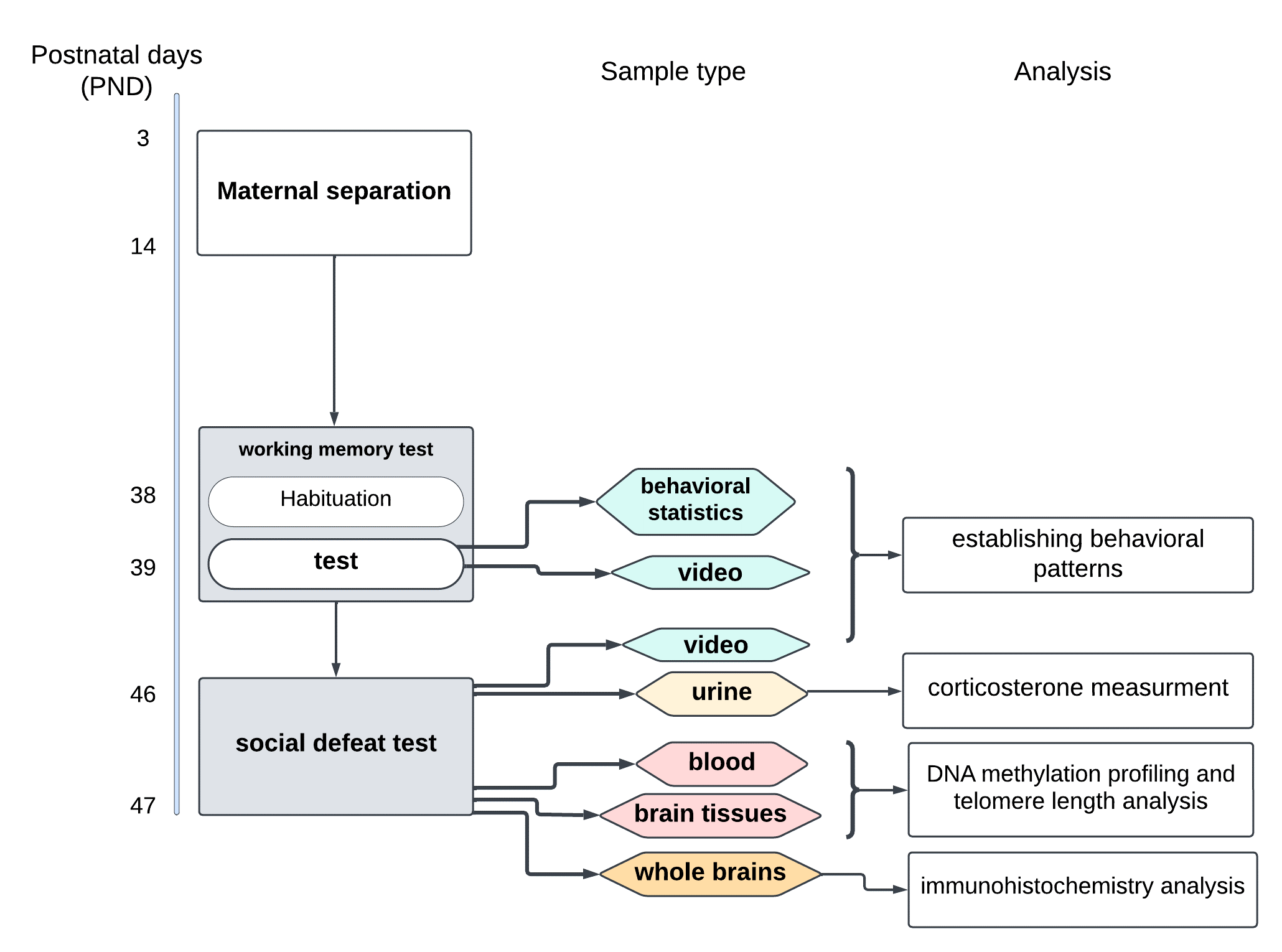
Fig. 2. The experiment roadmap. A) Dam with litter. At this stage, the number of pups in a litter is evaluated; litters with less than 3 pups are excluded from the protocol; B) During the MS, developmental milestones (eyes opening, ear positioning, and hair growth) are tracked; C) In radial maze test the number of re-entries in the baited arm for each animal is counted; D) In SD test the number and duration of attacks is noted, as well as specific submissive poses. Urine samples are collected and processed for the enzyme-linked immunosorbent assay (ELISA) to measure the amount of corticosterone in a sample; the ELISA is performed according to the protocol described in [4]; E) Blood sample is collected using cardiac puncture for subsequent DNA extraction followed by telomere length analysis using a quantitative real-time PCR (the protocol is described in [16]), and epigenotyping via genome-wide DNA methylation array. Prior to the brain tissue collection, the MS and control animals are randomly assigned to two groups for 2 different downstream analyses of the target brain regions — amygdala, hippocampus, and prefrontal cortex: (1) immunohistochemical analysis (see Methods, [29]) of GR density according to the manual described in [14], and (2) DNA extraction followed by the telomere length measurement and DNA methylation profiling using the above molecular genetic techniques.
Discussion
- While the first set of 4 litters undergoes MS, an order for 4 pregnant dams should be placed so they are delivered near the end of MS.
- When the first set is weaned and left to grow (for approximately 2 weeks), the second set of 4 litters is ready for MS.
- When the second set of litters is weaned and left to grow, the first set can perform behavioral experiments. Thus, the researchers have two weeks to conduct the maze test and the SD test and to sacrifice animals. Having completed this first round, they can engage the second set of mice. As a result, nine weeks are needed to process approximately 50 mice. This number can be increased if there are multiple rooms for MS, behavior rooms, and enough personnel.
Conclusion
References
- Golden S.A., Covington III H.E., Berton O., Russo S.J. A standardized protocol for repeated social defeat stress in mice. Nature Protocols, 2011. Vol. 6, no. 8, pp. 1183—1191. DOI:10.1038/nprot.2011.361
- Brajon S., Morello G.M., Capas-Peneda S., Hultgren J., Gilbert C., Olsson A. All the Pups We Cannot See: Cannibalism Masks Perinatal Death in Laboratory Mouse Breeding but Infanticide Is Rare. Animals, 2021. Vol. 11, no. 8, article ID 2327. 18 p. DOI:10.3390/ani11082327
- Murra D., Hilde K.L., Fitzpatrick A., Maras P.M., Watson S.J., Akil H. Characterizing the behavioral and neuroendocrine features of susceptibility and resilience to social stress. Neurobiology of Stress, 2022. Vol. 17, article ID 100437. 11 p. DOI:10.1016/j.ynstr.2022.100437
- Kim S., Foong D., Cooper M.S., Seibel M.J., Zhou H. Comparison of blood sampling methods for plasma corticosterone measurements in mice associated with minimal stress-related artefacts. Steroids, 2018. Vol. 135, pp. 69—72. DOI:10.1016/j.steroids.2018.03.004
- Rocha M., Wang D., Avila-Quintero V., Bloch M.H., Kaffman A. Deficits in hippocampal-dependent memory across different rodent models of early life stress: systematic review and meta-analysis. Translational Psychiatry, 2021. Vol. 11, no. 1, article ID 231. 12 p. DOI:10.1038/s41398-021-01352-4
- MacDowell C.J, Briones B.A, Lenzi M.J, Gustison M.L, Buschman T.J Differences in the expression of cortex-wide neural dynamics are related to behavioral phenotype. Current Biology, 2024. Vol. 34, pp. 1—8. DOI:10.1016/j.cub.2024.02.004
- Razzoli M., Carboni L., Andreoli M., Ballottari A., Arban R. Different susceptibility to social defeat stress of BalbC and C57BL6/J mice. Behavioural Brain Research, 2011. Vol. 216, no. 1, pp. 100—108. DOI:10.1016/j.bbr.2010.07.014
- Hisey E.E., Fritsch E.L., Newman E.L., Ressler K.J., Kangas B.D., Carlezon Jr. W.A. Early life stress in male mice blunts responsiveness in a translationally-relevant reward task. Neuropsychopharmacology, 2023. Vol. 48, pp. 1752—1759. DOI:10.1038/s41386-023-01610-7
- Touma C., Sachser N., Möstl E., Palme R. Effects of sex and time of day on metabolism and excretion of corticosterone in urine and feces of mice. General and Comparative Endocrinology, 2003. Vol. 130, no. 3, pp. 267—278. DOI:10.1016/S0016-6480(02)00620-2
- van Heerden J.H., Russell V., Korff A., Stein D.J., Illing N. Evaluating the behavioural consequences of early maternal separation in adult C57BL/6 mice; the importance of time. Behavioural Brain Research, 2010. Vol. 207, no. 2, pp. 332—342. DOI:10.1016/j.bbr.2009.10.015
- Newman E.L., Covington III H.E., Suh J., Bicakci M.B., Ressler K.J., DeBold J.F., Miczek K.A. Fighting Females: Neural and Behavioral Consequences of Social Defeat Stress in Female Mice. Biological psychiatry, 2019. Vol. 86, no. 9, pp. 657—668. DOI:10.1016/j.biopsych.2019.05.005
- Fox R.R., Witham B.A., Neleski L.A. Handbook on Genetically Standardized JAX Mice. 5th ed. Bar Harbor: Jackson Laboratory, 1997. 143 p.
- Heffner T.G., Hartman J.A., Seiden L.S. A rapid method for the regional dissection of the rat brain. Pharmacology Biochemistry and Behavior, 1980. Vol. 13, no. 3, pp. 453—456. DOI:10.1016/0091-3057(80)90254-3
- Stanojevic J., Dragic M., Stevanovic I., Ilic T., Stojanovic I., Zeljkovic M., Ninkovic M. Intermittent theta burst stimulation ameliorates cognitive impairment and hippocampal gliosis in the Streptozotocin-induced model of Alzheimer’s disease. Behavioural Brain Research, 2022. Vol. 433, article ID 113984. 15 p. DOI:10.1016/j.bbr.2022.113984
- Krauth J. The interpretation of significance tests for independent and dependent samples. Journal of Neuroscience Methods, 1983. Vol. 9, no. 4, pp. 269—281. DOI:10.1016/0165-0270(83)90058-4
- Sarıbal D., Aydın A.K., Kılıç M.A., Shakil F., Balkaya M. Maternal neglect results in reduced telomerase activity and increased oxidativeload in rats. Stress, 2021. Vol. 24, no. 3, pp. 348—352. DOI:10.1080/10253890.2020.1777973
- Wang H., van Leeuwen J.M.C., de Voogd L.D., Verkes R.-J., Roozendaal B., Fernández G., Hermans E.J. Mild early-life stress exaggerates the impact of acute stress on corticolimbic resting-state functional connectivity. European Journal of Neuroscience, 2022. Vol. 55, no. 9—10. pp. 2122—2141. DOI:10.1111/ejn.15538
- Kotlinska J.H., Grochecki P., Michalak A., Pankowska A., Kochalska K., Suder P., Ner-Kluza J., Matosiuk D., Marszalek-Grabska M. Neonatal Maternal Separation Induces Sexual Dimorphism in Brain Development: The Influence on Amino Acid Levels and Cognitive Disorders. Biomolecules, 2023. Vol. 13. no. 10, article ID 1449. 17 p. DOI:10.3390/biom13101449
- Possamai-Della T., Cararo J.H., Aguiar-Geraldo J.M., Peper-Nascimento J., Zugno A.I., Fries G.R., Quevedo J., Valvassori S.S. Prenatal Stress Induces Long-Term Behavioral Sex-Dependent Changes in Rats Offspring: the Role of the HPA Axis and Epigenetics. Molecular Neurobiology, 2023. Vol. 60, pp. 513—533. DOI:10.1007/s12035-023-03348-1
- Rahman M.F., McGowan P.O. Cell-type-specific epigenetic effects of early life stress on the brain. Translational Psychiatry, 2022. Vol. 12, article ID 326. 10 p. DOI:10.1038/s41398-022-02076-9
- Rentscher K.E., Carroll J.E., Mitchell C. Psychosocial Stressors and Telomere Length: A Current Review of the Science. Annual Review of Public Health, 2020. Vol. 41, pp. 223—245. DOI:10.1146/annurev-publhealth-040119-094239
- Holly E.N., Shimamoto A., DeBold J.F., Miczek K.A. Sex differences in behavioral and neural cross-sensitization and escalated cocaine taking as a result of episodic social defeat stress in rats. Psychopharmacology, 2012. Vol. 224, pp. 179—188. DOI:10.1007/s00213-012-2846-2
- Bonapersona V., Damsteegt R., Adams M.L., van Weert L.T.C.M., Meijer O.C., Joëls M., Sarabdjitsingh R.A. Sex-Dependent Modulation of Acute Stress Reactivity After Early Life Stress in Mice: Relevance of Mineralocorticoid Receptor Expression. Frontiers in Behavioral Neuroscience, 2019. Vol. 13, article ID 181. 15 p. DOI:10.3389/fnbeh.2019.00181
- Smith K.E., Pollak S.D. Early life stress and development: potential mechanisms for adverse outcomes. Journal Neurodevelopmental Disorder, 2020. Vol. 12, article ID 34. 15 p. DOI:10.1186/s11689-020-09337-y
- Jacobson-Pick S., Audet M.-C., McQuaid R.J., Kalvapalle R., Anisman H. Social Agonistic Distress in Male and Female Mice: Changes of Behavior and Brain Monoamine Functioning in Relation to Acute and Chronic Challenges. PLoS One, 2013. Vol. 8, no. 4, article ID e60133. 17 p. DOI:10.1371/journal.pone.0060133
- Stengel A., Wang L., Taché Y. Stress-related alterations of acyl and desacyl ghrelin circulating levels: Mechanisms and functional implications. Peptides, 2011. Vol. 32, no. 11, pp. 2208—2217. DOI:10.1016/j.peptides.2011.07.002
- Whitaker J., Moy S.S., Saville B.R., Godfrey V., Nielsen J., Bellinger D., Bradfield J. The effect of cage size on reproductive performance and behavior of C57BL/6 mice. Lab Animal, 2007. Vol. 36, no. 10, pp. 32—39. DOI:10.1038/laban1107-32
- Thorpe J.B., Rajabi N., deCatanzaro D. Circadian Rhythm and Response to an Acute Stressor of Urinary Corticosterone, Testosterone, and Creatinine in Adult Male Mice. Hormone and Metabolic Research, 2012. Vol. 44, no. 06, pp. 429—435. DOI:10.1055/s-0032-1306307
- Wu J., Cai Y., Wu X., Ying Y., Tai Y., He M. Transcardiac Perfusion of the Mouse for Brain Tissue Dissection and Fixation. Bio Protocol, 2021. Vol. 11, no. 5, article ID e3988. 11 p. DOI:10.21769/BioProtoc.3988
- Trask S., Kuczajda M.T., Ferrara N.C. The lifetime impact of stress on fear regulation and cortical function. Neuropharmacology, 2023. Vol. 224, article ID 109367. 11 p. DOI:10.1016/j.neuropharm.2022.109367
- Hasegawa A., Mochida K., Nakamura A., Miyagasako R., Ohtsuka M., Hatakeyama M., Ogura A. Use of anti-inhibin monoclonal antibody for increasing the litter size of mouse strains and its application to in vivo-genome editing technology. Biology of Reproduction, 2022. Vol. 107, no. 2, pp. 605—618. DOI:10.1093/biolre/ioac068
- Ródenas-González F., Arenas M.C., Blanco-Gandía M.C., Manzanedo C., Rodríguez-Arias M. Vicarious Social Defeat Increases Conditioned Rewarding Effects of Cocaine and Ethanol Intake in Female Mice. Biomedicines, 2023. Vol. 11, no. 2, article ID 502. 22 p. DOI:10.3390/biomedicines11020502
- Lin L., Wu J., Yuan Y., Sun X., Zhang L. Working Memory Predicts Hypothalamus-Pituitary-Adrenal Axis Response to Psychosocial Stress in Males. Frontiers in Psychiatry, 2020. Vol. 11, article ID 142. 9 p. DOI:10.3389/fpsyt.2020.00142
Information About the Authors
Metrics
Views
Total: 33
Previous month: 18
Current month: 15
Downloads
Total: 12
Previous month: 4
Current month: 8