INTRODUCTION
Low-grade systemic inflammation is a persistent condition characterized by subclinical activation of systemic immunoinflammatory processes [1, 2]. It is known that systemic inflammation is involved in the pathophysiology of cardiovascular [3], endocrine [4], dermatological [5], oncological [6], and neurological diseases [7]. There is also evidence of activation of immune and inflammatory mechanisms in mental disorders such as depression [8–10], schizophrenia [8, 11, 12], and anxiety disorders [13]. Genetic predisposition, early life adversity, acute or chronic stress, unhealthy diet, and changes in the microbiome all contribute to this activation [1, 9, 14]. Systemic inflammation might influence the course of mental disorders, their clinical features, and severity of psychopathological symptoms [15–17]. An association between systemic inflammation and treatment therapeutic resistance has been established [18, 19]. In addition, systemic inflammation may be one of the common pathogenetic links between mental disorders and the metabolic syndrome, contributing to their frequent comorbidity [20, 21].
Peripheral blood levels of pro- and anti-inflammatory cytokines are usually considered as biomarkers of systemic inflammation in various mental disorders [22, 23]. Inflammatory hematological ratios (IHRs), such as the neutrophil to lymphocyte ratio (NLR), monocyte to lymphocyte ratio (MLR), platelet to lymphocyte ratio (PLR) can serve as inexpensive and readily available biomarkers [24, 25]. The above listed ratios, which characterize both innate and acquired immunity [26], have been studied as risk factors and/or predictors of the severity of COVID-19 [27], oncological [28], endocrine [29], and cardiovascular disorders [30, 31].
The rationale for studying IHRs in the context of mental disorders stems from the involvement of certain immune cells in the pathological processes associated with inflammation. One of the reasons for a decrease in the number of lymphocytes relative to other cells, in particular neutrophils, may be an increase in catecholamines, as well as in the blood prolactin and cortisol levels, which is observed, for example, under stress. There is evidence that monocytes can enter the central nervous system (CNS) and increase neuroinflammation, which, combined with a potential decrease in the lymphocyte count, justifies interest in the ratio of these cells. Platelets contain pro-inflammatory factors (metalloproteinases, chemokines, cytokines, etc.) and can be involved in an increase in the permeability of the blood-brain barrier and the regulation of inflammation in the CNS, which suggests that it is important to study their number relative to other cells; in particular, lymphocytes [32]. Elevated IHRs have been observed in patients with schizophrenia [33–35] and affective disorders [36, 37]. Research into the relationship between IHRs and schizophrenia is summarized in the scoping review that includes the results of 13 studies, predominantly in adult patients [38]. Given that many chronic and recurring mental disorders manifest themselves in adolescence [39, 40], systematizing IHRs studies in this age group is important. Moreover, many mental disorders in adolescence are “transdiagnostic” [41], posing challenges for their diagnosis and prognosis [42–44].
The aim of this scoping review was to summarize the findings from the studies that investigated the relationship of IHRs with mental disorders in adolescent patients. The following study questions were addressed in this review: 1) In what mental disorders in adolescents is there a difference in IHRs compared with healthy individuals of the same age? Have IHRs been examined as diagnostic biomarkers of mental disorders in adolescence (with cut-off values, sensitivity, and specificity calculations)? 2) Is there an association between IHRs and clinical features reflecting more severe/acute manifestations of mental disorders (severity of symptoms, acute phase of the disorder, presence of self-harm/suicidal behaviors), as well as the treatment response and the components of the metabolic syndrome? Have IHRs been examined as prognostic biomarkers for these variables (with cut-off values, sensitivity, and specificity calculations)?
METHODS
Protocol and registration
The aim of this scoping review, eligibility criteria, and methods for this review were defined in a protocol which is available upon request addressed to the corresponding author. The protocol was not registered in a public database. No changes were made to the protocol during the study (search, data extraction, and analysis). No deviations from the protocol were identified.
Eligibility criteria
The review included original studies that:
- Were conducted in adolescents (aged 10 to 19 years inclusive) with mental disorders;
- Assessed ihrs as a studied parameter (study factor);
- Were published in english; and
- Were published before December 31, 2023.
The following studies were not included:
- Those with mixed-age samples (younger children and adolescents, adolescents and adults); and
- Those that assessed IHRs in anorexia nervosa (criterion is justified by the likely influence of undernutrition on the activity of immune inflammation [45]).
Information sources
The search for information sources was carried out in the electronic database MEDLINE (access via PubMed1). The final search was conducted on January 16, 2024.
Search
To identify potentially relevant sources, a search query was used, which was generated through the following steps:
- Identifying 3 primary concepts consistent with the aim of the review: IHRs, adolescence, mental disorders;
- Expanding these concepts with relevant synonyms;
- Combining keywords using boolean operators;
- Finalizing the search query based on the result of a discussion and consensus amongst all authors after a pilot search in the MEDLINE electronic database: (blood count parameters) OR (inflammatory ratios) OR (lymphocyte monocyte ratio) OR (platelet lymphocyte ratio) OR (systemic immune inflammation index) OR (monocyte-to-high-density lipoprotein ratio) AND (adolescents) AND (mental disorders) OR (depression) OR (suicide) OR (schizophrenia) OR (bipolar disorder).
The search was conducted by one of the authors (OL).
Selection of sources of evidence
The selection of publications from the identified sources was carried out in 3 stages:
- Screening by titles and abstracts to exclude obviously irrelevant sources of information (e.g. In vitro studies, studies on laboratory animals, studies that included only adults);
- Full texts retrieval; and
- Analysis of the retrieved full-text sources using the eligibility criteria indicated above.
If the inclusion criteria were met and no exclusion criteria were met, studies were selected for inclusion in the review regardless of their design. Sources were selected independently by two authors (MP and OL). Discrepancies identified during comparison were corrected through discussion and consensus-building amongst all authors.
Data charting process
Data were extracted from the selected publications according to a pre-designed data collection form. Data were charted by one of the authors (OL) and subsequently cross-checked by another author (MP). Inconsistencies were discussed by all authors. All identified discrepancies were of a technical nature. There were no major discrepancies.
Data items
The following data were extracted: authors, country, year of publication, study design, diagnoses and diagnostic criteria, age, sample size, sex distribution of participants, presence/absence of treatment, study setting (inpatient or outpatient), IHR values (any parameters were extracted – all ratios calculated by the authors of the original papers based on hematology data), and the statistical significance of the differences compared with the control group. If there were healthy controls in the study, data from both the patients and the healthy participants were extracted.
Additionally, we extracted the findings regarding relationship between IHRs and the severity of symptoms, the disease phase, the presence of self-harm/suicidal behaviors, the treatment response, the components of the metabolic syndrome (body mass index, waist circumference, blood pressure, plasma glucose and glycated hemoglobin levels, lipid profile), as well as the results of the Receiver Operator Characteristic (ROC) curve analysis with IHRs cut-off values, sensitivity, and specificity (if the source contained these data).
Critical appraisal of individual sources of evidence
Not performed.
Synthesis of results
All relevant publications were analyzed after being assigned to one of the two groups. The first group included studies that compared IHR values between adolescents with a mental disorder and healthy individuals of the same age (healthy controls). The second group included studies that examined the relationship between IHRs and clinical variables (such as the phase of the disease, the presence of self-harm/suicidal behaviors). Data extracted from publications within each group were tabulated. Statistical methods were not used to analyze the data.
RESULTS
Selection of sources of evidence
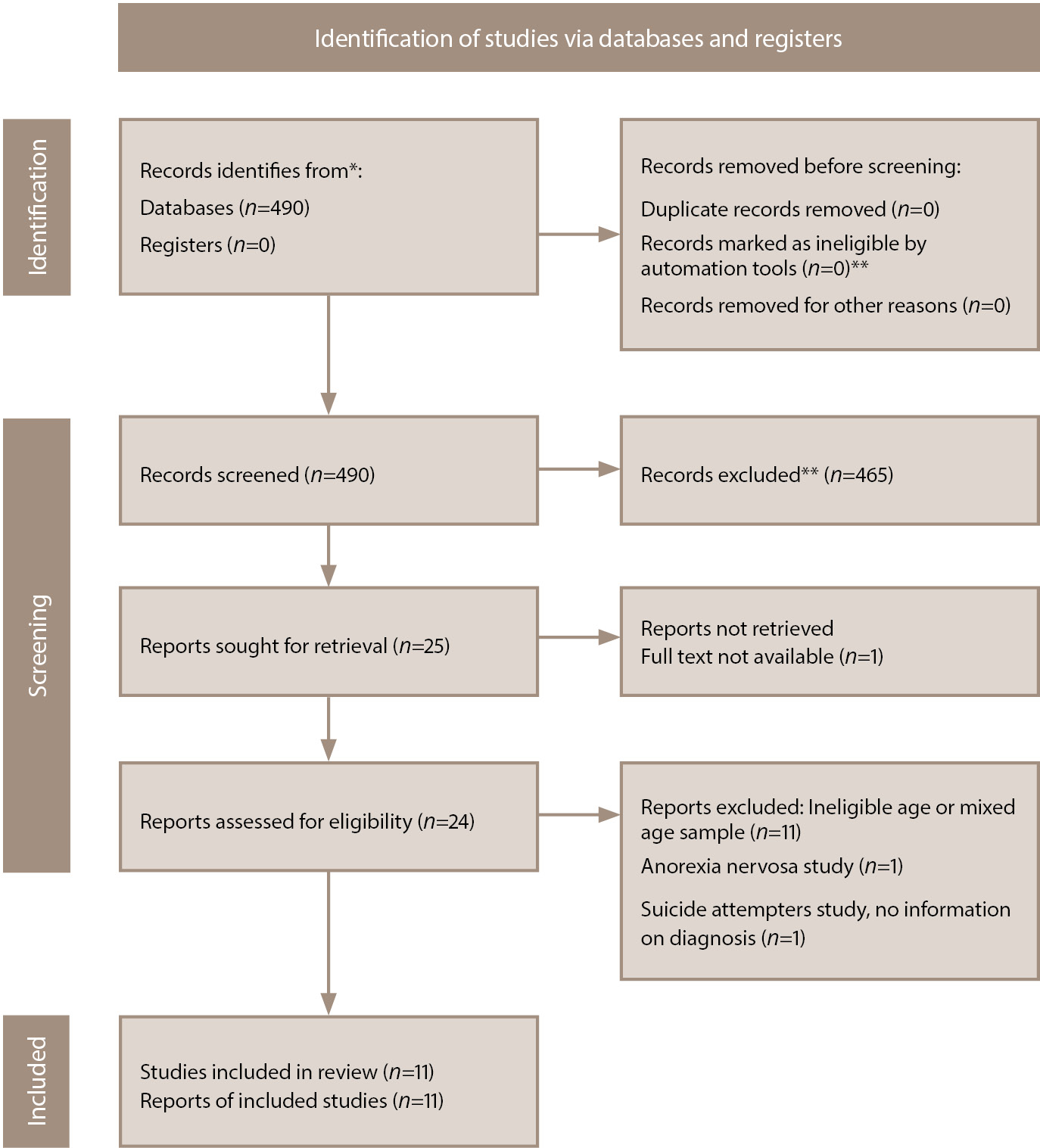
The search query identified 490 publications. After reviewing the titles of the articles and their abstracts, 465 publications were excluded as not relevant to the scope of the review (the reasons for the exclusion of each source were not recorded at this stage). Of the remaining publications, 1 was excluded from the review due to the unavailability of the full text. After reviewing the full texts of 24 articles, we included 11 publications in the review [46–56]. The main reason for exclusion from analysis was that the studies were ineligible due to the age of the participants (Figure 1).
Figure 1. PRISMA flow diagram [57] of the literature search and the selection process. Note: *All records were identified from MEDLINE database search (accessed via PubMed). **Automation tools were not used in this study. Records were excluded by a human.
Characteristics of sources of evidence
The articles selected for the review were published between 2018 and 2023. All the publications included original studies. Geographically, 6 studies were conducted in Turkey [47–52], 2 in China [53, 54], 2 in Israel [55, 56], and 1 in Slovakia [46]. Six studies examined data from adolescents with affective disorders: 2 studies included patients with bipolar disorder (BD) [50, 52], 2 studies included patients with major depressive disorder (MDD) [51, 53], 1 study included patients with affective episodes of BD/MDD [55], and 1 study included patients with various types of affective disorders [54]. One study included adolescents with psychotic disorders [56]; 1 — obsessive-compulsive disorder (OCD) [49]; 1 — attention-deficit hyperactivity disorder (ADHD) [47]; and 1 — substance use disorders (SUD) [48]. Another study examined 2 groups of adolescents with autism spectrum disorders (ASD) and with ADHD [46]. All included studies were cross-sectional. The characteristics of the studies included in the review, as well as their main results, are presented in Tables 1 and 2. The description of the study designs in the Tables is indicated as per original sources.
Table 1. Comparison of inflammatory hematological ratios in adolescents with psychiatric disorders vs healthy controls
|
Reference |
Country |
Type of study |
Diagnosis/ diagnostic criteria |
Eligible age, range |
Patients |
Healthy controls |
p-value |
|||||||
|
n |
Average age* |
Gender (M/F) |
Treatment/ setting |
IHR* |
n |
Average age* |
Gender (M/F) |
IHR* |
||||||
|
Ferencova, et al., 2023 [46] |
Slovakia |
Cross-sectional |
ASD/ DSM-5 |
10–19 |
20 |
12.4 (1.9) |
15/5 |
Naive/ inpatient |
NLR 1.15 (1.02–1.43) MLR 0.20 (0.15, 0.28) PLR 113.0 (99.7–119.0) LMR 4.92 (3.64–6.52) PMR 409 (370–590) |
20 |
13.2 (1.9) |
15/5 |
NLR 0.97 (0.80-1.37) MLR0.19 (0.16-0.22) PLR 103.0 (85.4-113.0) LMR 5.23 (4.56-6.45) PMR 531 (466-688) |
>0.05 (all variables) |
|
ADHD/ DSM-5 |
10–19 |
20 |
13.4 (1.8) |
15/5 |
Naive/ inpatient |
NLR 1.21 (0.91-1.73) MLR 0.19 (0.14–0.24) PLR 102.0 (72.1–125.0) LMR 5.22 (4.17–7.05) PMR 583 (415–700) |
||||||||
|
Önder, et al., 2021 [47] |
Turkey |
Retrospective cross-sectional |
ADHD/ DSM-5 |
12–17 |
24 |
NR |
NR |
Mixed (both naive and treated)/ outpatient |
NLR 1.95 (0.81) PLR 132.50 (42.35) |
29 |
NR |
NR |
NLR 1.16 (0.38) PLR 97.57 (18.37) |
<0.001 (NLR) 0.001 (PLR) |
|
Karatoprak, et al., 2021 [48] |
Turkey |
Retrospective cross-sectional |
SUD/ DSM-5 |
12–18 |
55 |
17 (16−17) |
55/0 |
Naive/ outpatient |
NLR 1.65 (1.17−2.18) PLR 111.05 (86.11−130.62) |
61 |
17 (16−17) |
61/0 |
NLR 1.44 (1.10−1.68) PLR 92.39 (83.75−107.28) |
0.006 (NLR) 0.007 (PLR) |
|
Özyurt, et al., 2019 [49] |
Turkey |
Cross-sectional |
OCD/ DSM-5 |
12–18 |
60 |
14.61 (1.81) |
17/43 |
Naive/ outpatient |
NLR 190.91 (148.00) PLR 118.02 (49.96) |
128 |
14.37 (1.80) |
27/101 |
NLR 148.81 (0.73) PLR 104.61 (44.66) |
0.003 (NLR) 0.004 (PLR) |
|
Ceylan, et al., 2019 [50] |
Turkey |
Cross-sectional |
BD-type I (remission)/DSM-5 |
14–17 |
38 |
16.3 (0.9) |
10/28 |
NR/ outpatient |
LMR 4.71 (1.7) NLR 1.84 (0.8) |
37 |
16.8 (0.6) |
10/27 |
LMR 5.57 (1.5) NLR 1.80 (0.6) |
0.54 (LMR) 0.75 (PLR) |
|
Özyurt, et al., 2018 [51] |
Turkey |
Cross-sectional |
MDD/ DSM-5 |
12–18 |
67 |
14.47 (1.85) |
20/47 |
Naive/ outpatient |
NLR 2.1 (1.1) PLR 127.14 (35.26) |
121 |
14.46 (1.77) |
26/95 |
NLR 1.59 (0.57) PLR 113.3 (36.86) |
<0.001 (NLR) 0.005 (PLR) |
|
Binici, et al., 2018 [52] |
Turkey |
Cross-sectional |
BD-type I (remission)/DSM-IV-TR |
NR |
36 |
16.42 (1.29) |
15/21 |
Treated/ outpatient |
NLR 1.9 (0.78) PLR 107.03 (32.04) |
30 |
16.3 (1.19) |
16/14 |
NLR 1.72 (0.77) PLR 122.35 (32.04) |
0.38 (NLR) 0.05 (PLR) |
Note: ADHD — attention deficit/hyperactivity disorder; ASD — autism spectrum disorders; BD-type I — bipolar disorder, type I; DSM-IV-TR — Diagnostic and Statistical Manual of mental disorders, fourth edition, text revision; DSM-5 — Diagnostic and Statistical Manual of mental disorders, fifth edition; IHR — inflammatory hematological ratios; LMR — lymphocyte to monocyte ratio; MDD — major depressive disorder; MLR — monocyte to lymphocyte ratio; NLR — neutrophil to lymphocyte ratio; NR — not reported; OCD — obsessive-compulsive disorder; PLR — platelet to lymphocyte ratio; PMR— platelet to monocyte ratio; SUD — substance use disorders. *Data is presented in the following way: mean (standard deviation), if one value in parentheses; median (interquartile range), if two values in parentheses separated by a dash.
Table 2. Comparison of inflammatory hematological ratios in adolescents with psychiatric disorders with regard to clinical features
|
Reference |
Country |
Type of study |
Diagnosis/ diagnostic criteria |
Eligible age, range |
Treatment/ setting |
Patients |
Controls (comparison group of patients) |
p-value |
||||||||
|
Clinical feature under study |
n |
Average age* |
Gender (M/F) |
IHR* |
Clinical feature under study |
n |
Average age* |
Gender (M/F) |
IHR* |
|||||||
|
Cui, et al, 2023 [53] |
China |
Retrospective cross-sectional |
MDD/ ICD-10 |
10–18 |
Naive/ inpatient |
Suicide attempt |
38 |
14.87 (1.89) |
9/29 |
SII index 537.49 (261.62) |
No suicide attempt |
225 |
14.75 (1.78) |
76/149 |
SII index 396,92 (200.68) |
0.002 |
|
Zheng, et al., 2022 [54] |
China |
Cross-sectional |
Mood or emotional disorders/ ICD-10 |
13–18 |
Treated/ inpatient |
NSSI, DSM-5 |
106 |
15 (median) |
17/89 |
NLR 1.46 (0.33) MLR 0.19 (0.01) PLR 115.66 (3.4) |
No NSSI, DSM-5 |
95 |
15 (median) |
21/74 |
NLR 1.34 (0.53) MLR 0.16 (0.04) PLR 107.85 (17.52) |
0.091 (NLR) 0.001 (MLR) 0.007 (PLR) |
|
Drapisz, et al., 2022 [55] |
Israel |
Retrospective cross-sectional |
Major affective episodes/ DSM-5 |
10–19 |
Treated/ inpatient |
Manic episode |
63 |
15.9 (1.6) |
38/25 |
NLR 2.36 (1.7) |
Depressive episode |
242 |
15.1 (1.8) |
147/95 |
NLR 1.87 (1.00) |
0.001 |
|
Manic episode |
13 |
14.9 (mean) |
6/7 |
NLR 2.00 (0.8) |
Remission |
13 ** |
15.6 (mean) |
6/7 |
NLR 1.50 (0.5) |
0.001 |
||||||
|
Bustan, et al., 2018 [56] |
Israel |
Retrospective cross-sectional |
Patients hospitalized in the acute ward without evidence of affective episodes (depressive, manic, hypomanic or mixed episodes)/ DSM-5 |
10–19 |
Treated/ inpatient |
Psychotic |
81 |
15.9 (1.6) |
47/34 |
NLR 2.51 (1.8) |
Non psychotic |
285 |
14.7 (1.8) |
147/138 |
NLR 1.91 (1.0) |
0.001 |
|
Acute psychosis |
20 |
15.9 (mean) |
NR |
NLR 2.65 (2.0) |
Remission |
20 ** |
16.3 (mean) |
NR |
NLR 1.74 (0.8) |
0.048 |
||||||
Note: DSM-5 — Diagnostic and Statistical Manual of mental disorders, fifth edition; ICD-10 — International Classification of Diseases, 10th revision; IHR — inflammatory hematological ratios; MDD — major depressive disorder; MLR — monocyte to lymphocyte ratio; NLR — neutrophil to lymphocyte ratio; NR — not reported; NSSI — non-suicidal self-injury; PLR — platelet to lymphocyte ratio; SII — systemic immune-inflammation.
*Data is presented in the following way, unless otherwise stated: mean (standard deviation). ** Same patients as in the Patients group.
Results of individual sources of evidence
Comparison of adolescents with mental disorders with healthy controls
IHR values in adolescents with mental disorders and healthy adolescents were compared in 7 [46–52] out of the 11 studies (see Table 1). In these studies, higher NLR and PLR values were found in adolescents with ADHD [47], substance use disorders [48], MDD [51], and OCD [49]. In the latter study, the NLR values were many times higher (approximately 100 times) than in other studies, which may be the result of a technical error on the part of the authors of the original article (for more details, see below, section “Limitations”). No statistically significant differences in IHRs between patients and healthy individuals were found in the studies of adolescents with BD [50, 52], as well as in the study that included 2 samples — adolescents with ASD and ADHD [46].
Diagnostic value of IHRs
None of the 7 studies listed above assessed the diagnostic value of IHRs (cut-off values, sensitivity, and specificity were not reported).
Association between IHRs and the severity of symptoms of mental disorders
The association between IHRs and the severity of psychopathological symptoms was examined in 2 studies [47, 51]. In adolescents with ADHD, no association was found between IHRs and the symptoms severity [47]. In adolescents with MDD, NLR values were positively correlated with the Beck’s Depression Inventory score and the disease duration [51]. It is worth noting that in adolescents with BD, neither NLR nor PLR were correlated with the duration of the disorder or age of its onset [52]. Adolescents with OCD and comorbid anxiety disorders (which indirectly suggests a greater severity of the disorder) had higher NLR compared with OCD without comorbidity [49].
Association between IHRs and other clinical features of mental disorders
Four studies [53–56] investigated the association between IHRs and certain clinical features of a mental disorder (see Table 2). One study demonstrated a statistically significant increase in the systemic immune-inflammation index (SII, the product of platelet and neutrophil counts divided by the lymphocyte count) in adolescents with MDD who had attempted suicide compared with patients with MDD without a history of suicide attempts [53]. Another study found a significant increase in MLR and PLR in adolescents with non-suicidal self-injuries in affective disorders compared with similar patients without self-injuries [54]. In the third study, higher NLR values were observed in adolescents in a manic episode of BD than in a depressive episode [55]. Additionally, a significant decrease in NLR in remission was observed compared with a manic episode (mean interval between blood tests was 264 days) [55]. Finally, the fourth study demonstrated that the mean value of NLR in adolescents with psychotic disorders (mainly schizophrenia spectrum) was higher than in non-psychotic patients (with conduct disorders, adjustment disorder, ADHD) [56]. The same study showed a decrease in NLR after patients had achieved clinical remission compared with an acute psychotic state (mean interval between blood tests was 157 days) [56].
Prognostic value of IHRs
Although a ROC analysis was conducted in two studies that included adolescents with self-harm/suicidal behaviors to determine the IHR cut-off values, their sensitivity and specificity [53, 54], the results did not allow us to assess the prognostic value of IHRs. In the study that included adolescents with MDD — with or without a history of suicide attempts [53] — the area under the curve for the SII index was 0.661 (95% confidence interval, CI, 0.550–0.772; p=0.002), the optimal cut-off value for the SII index (based on the maximum value of Youden’s index) was 548.15, with a sensitivity of 63% and specificity of 83%. Based on this cut-off value, patients were divided into high and low SII groups and a binary logistic regression analysis was performed. After adjusting for sex, age, body mass index, illness duration, and Hamilton Depression Rating Scale score, the odds of a suicide attempt within the last 7 days in the group of adolescents with high SII index were almost 14 times higher compared with the group of patients with SII index below the cut-off (odds ratio, OR=13.92; 95% CI 5.60–34.69; p <0.001). At the same time, a high SII index was not associated with a suicide attempt more than 7 days prior (OR=0.55; 95% CI 0.06–4.84; p=0.587) [53]. For non-suicidal self-injury in patients with affective disorders [54], the area under the curve was 0.638 (95% CI 0.561–0.715; p <0.001) for MLR and 0.611 (95% CI 0.533–0.689; p <0.001) for PLR. The cut-off values calculated by the authors of the original study were 0.135 for MLR (sensitivity 91%, specificity 34%) and 127.5 for PLR (sensitivity 40%, specificity 81%) [54]. It should be emphasized that although the authors of these studies indicate the association between increased “risk” of self-harm/suicidal behaviors and higher IHR values, this conclusion is based on data from retrospective cross-sectional studies, which completely excludes the possibility of assessing the prognostic value of IHRs (for more details, see below, section “Limitations”).
Association between IHRs and metabolic disturbances
The relationship between IHRs and the metabolic syndrome was not examined in the studies included in this review. In one study, which included adolescents with BD, no correlations between NLR or PLR and body mass index were found [52].
Association between IHRs and the treatment response
None of the studies included in this review examined the association between IHRs and the treatment response in mental disorders. One study revealed no differences in NLR or PLR in adolescents with ADHD who did and did not receive pharmacological treatment for their disorder, as well as no correlation of either NLR or PLR with the duration of atomoxetine and/or methylphenidate use [47].
DISCUSSION
Summary of evidence
Our search strategy did not identify any narrative reviews, scoping reviews, systematic reviews, or meta-analyses that systematized studies on the relationship between IHRs and mental disorders in adolescents. Having summarized the findings from 11 original studies selected for this scoping review, we can state the following. First, adolescents with mental disorders (depression, psychotic disorders, OCD, ADHD, substance use disorders) have higher IHRs compared with adolescents without these disorders. Second, IHRs are higher in adolescents with more severe/acute manifestations of the mental disorder (severity of symptoms, mania, exacerbation of psychosis, self-harm/suicidal behaviors). Third, the study results do not allow for the assessment of the diagnostic or prognostic value of IHRs in adolescents with mental disorders.
Limitations
The studies included in our review demonstrated heterogeneity (demographic and clinical characteristics of participants, different diagnoses, study settings, presence/absence of treatment, sample sizes). Although we did not assess the quality of the selected studies, several evident shortcomings are notable. In particular, most of the studies lack information on the procedures of blood collection and hematological analysis. In one study [49], the NLR values in both the patient and control groups were approximately 100 times higher than in other studies. The authors of the original article do not explain this in any way. Additionally, the existing discrepancy between the mean NLR value and its standard deviation in the control group (a difference of approximately two decimal orders, see Table 1) indicates a possible technical error (typo). However, such errors, combined with the above-mentioned heterogeneity of the studies, limit the comparability and generalizability of the results.
All the studies included in the review, according to their authors, were cross-sectional, which makes it impossible to establish causal relationships. Only 2 of these studies included a longitudinal (retrospective) part [55, 56], allowing to track the changes in the variables under study across time in some patients. About half of the studies were retrospective, raising concerns about the quality of the data that the study authors extracted from medical records not initially intended for study purposes. Both of the studies that performed an ROC analysis to calculate IHR cut-off values, sensitivity, and specificity were retrospective cross-sectional [53, 54]. Although the authors of these studies related high IHR values to the “risks” of self-harm/suicidal behaviors (suicide attempts and non-suicidal self-injury), those “risks” corresponds solely to past behaviors, precluding an assessment of the prognostic value of the suggested statistical models.
This review did not consider any other markers of inflammation, which prevents one from drawing conclusions about whether IHRs are independent indicators of systemic inflammation or are related to other immune inflammatory changes associated with mental disorders. This limitation precludes the possibility of assessing the influence of age on the associations of IHRs with other immune inflammatory markers.
Finally, some relevant studies may have been missed for the following reasons. First, the search for sources was limited to one database. Second, the search query used may not have been sensitive enough. Third, auxiliary search methods were not used, in particular, in searching through reference lists in the relevant sources and other work published on the topic that used a systematic literature search methodology. For example, a published retrospective study of IHRs in 32 adolescents with early-onset schizophrenia was identified after the completion of the selection of information sources [58]. The reason for the omission was that the publication was not indexed in the MEDLINE database in which the search was conducted. The omitted study showed higher NLR in adolescents with schizophrenia compared with healthy controls of the same age, which is consistent with the results of the study included in our review demonstrating elevated NLR in adolescents with psychotic disorders (including schizophrenia) compared with non-psychotic adolescents [56].
Discussion of the main results in comparison with the results of IHR studies in adults and younger children
Association between IHRs and mental disorders
Several systematic reviews and meta-analyses have been published summarizing data on IHRs in mental disorders across various age groups, including children [25, 59], adults [33, 36, 37, 60, 61], and mixed-age samples [38]. The majority of these studies focus on affective disorders in adult patients.
A meta-analysis of the results of 7 studies on IHRs in BD in adults (1,334 participants) demonstrated that patients had higher NLR and PLR than healthy individuals: standardized mean difference, SMD=0.672; 95% CI 0.516–0.828; p <0.001 and SMD=0.425; 95% CI 0.004–0.846; and p=0.048, respectively [36], reflecting a moderate effect size. The results of 2 studies of BD in adolescents included in our review did not show differences in IHRs compared with the healthy controls [50, 52]. However, both adolescent studies included patients in remission, and, given this, their results are entirely consistent with the adult studies on BD which also included patients in remission and similarly revealed no differences from healthy controls [36].
A meta-analysis of the results of 4 studies on IHRs in MDD (553 participants) demonstrated higher NLR in adult patients compared with healthy controls (SMD=0.670; 95% CI 0.072–1.268; p=0.028) [36]. A meta-analysis of the results of studies examining any relationship between IHRs and depression (2,580 adult patients with depression and 2,664 healthy participants) allowed us to draw similar conclusions: higher NLR in depressive patients than in healthy controls (SMD=0.33; 95% CI 0.15–0.45; p <0.001) and no differences in PLR or MLR [60]. Another meta-analysis (18 studies, 2,264 adults with depression and 2,415 healthy participants) confirmed the increase in NLR (SMD=0.33; 95% CI 0.15–0.52; p <0.001) and PLR (SMD=0.24; 95% CI 0.02–0.46; p <0.05) in depression compared with healthy individuals [37]. All these results are consistent with the results of the study included in our review [51], which found higher NLR and PLR in adolescents with MDD compared with healthy individuals of the same age.
A meta-analysis of studies on IHRs in psychotic disorders in adults (8 studies, 3 of which included patients with the first psychosis episode and 5 with schizophrenia; a total of 683 patients and 551 healthy participants) demonstrated that patients with non-affective psychosis had higher NLR and MLR than healthy controls (SMD=0.715; 95% CI 0.525–0.905; p <0.001 and SMD=0.417; 95% CI 0.147–0.686; p=0.002, respectively) [33]. A study in adolescents with acute psychotic disorders included in our review also found an increase in NLR compared with healthy adolescents (MLR was not assessed in that study) [56]. The increase in NLR in adolescents with psychotic disorders compared with non-psychotic adolescents is also confirmed by a meta-analysis of the results of 3 studies in this age group including 557 participants [25].
A meta-analysis of the results of 8 studies on IHRs in younger children with ADHD (mean age of participants of these studies varied from 8.3±1.7 to 10.33±3.15 years) demonstrated that they had higher NLR and PLR than healthy children (939 patients and 652 healthy children; SMD=0.49; 95% CI 0.15–0.82; p=0.004 and SMD=0.31; 95% CI 0.03–0.59, respectively), while no difference in MLR was observed [59]. The results of the studies in adolescents included in our review were inconsistent: one study demonstrated increased NLR and PLR in adolescents aged 12–17 years with ADHD [47], while another found no differences compared with healthy controls [46].
As for other mental disorders (which have been studied in adolescents), elevated IHRs compared with healthy controls were demonstrated in adult patients with OCD [62, 63] and SUD [64, 65], and in children with ASD [66–68]. It should be noted that the number of such studies is limited and their results are somewhat contradictory, which makes comparisons extremely difficult, especially regarding the age-specificity.
Overall, a comparison of studies on IHRs in mental disorders between adolescents and adults indicates that the most reproducible abnormalities compared with healthy individuals in both age groups are NLR and (to a lesser extent) PLR increase in affective disorders [36, 37, 51, 60], as well as NLR increase in schizophrenia spectrum disorders (first psychotic episode and schizophrenia) [25, 33, 56]. Comparison of studies of IHRs in ADHD between adolescents [46, 47] and younger children [59] demonstrates an increase in NLR compared with healthy controls (although not in all studies) in both age groups. Thus, for those indications which were studied across various age groups (younger children, adolescents, adults), we did not find any age-related differences in the association between IHRs and mental disorder.
Association between IHRs and the clinical features of mental disorders
Relationships between IHRs and the severity of psychopathological symptoms have been investigated in a few studies. One of the studies included in our review demonstrated a correlation between NLR and the severity of depressive symptoms in adolescents with MDD [51]. In adults, the severity of depression correlated for stronger with PLR than NLR [69, 70], which may indicate age-related differences in the relationship between IHRs and the severity of depressive symptoms. A correlation between NLR and symptom severity has been observed in adult patients with schizophrenia [71]. In ADHD, no correlations between IHRs and symptom severity have been found in either adolescents [47] or younger children [72]. Given the limited number of studies, it is difficult to determine the age-specific differences in the relationship between IHRs and the severity of psychopathological symptoms. Therefore, further studies are needed to confirm the reproducibility of the relevant findings.
In the studies investigating the relationship between IHRs and the illness phase, higher NLR values were observed in adolescents in a manic episode of BD than in a depressive episode or remission [55], as well as in adolescents with an acute psychosis compared with remission [56], which is fully consistent with the results of the studies in adults with BD [36, 73, 74] and psychotic disorders, including schizophrenia [33, 75]. These data confirm that higher IHR values are associated with more acute manifestations (mania, exacerbation of psychosis). However, no conclusions about the age-specificity in the relationship between IHRs and the disease phase can be drawn.
One of the important “indicators” of the acuity/severity in psychiatry is self-harm/suicidal behaviors. A study included in our review demonstrated an increase in the SII index in adolescents with MDD who attempted suicide compared with adolescents with MDD without suicide attempts [53]. Another study (193 adolescents aged 11–18 years with a history of suicide attempts and 109 non-suicidal participants of the same age), excluded from our review due to the lack of information regarding psychiatric diagnoses of study participants, demonstrated the association between suicidality and higher NLR, MLR, and PLR values [76]. In a sample of young adults (137 patients with MDD aged 18 to 24 years and 56 healthy controls of the same age), suicidality was associated with higher MLR values [77]. In adults, a systematic review of 11 studies (819 patients with MDD and suicidal behavior, 494 patients with MDD without suicidal behavior, and 388 healthy participants) revealed that suicidal behavior was associated with increased NLR, but not MLR or PLR [61]. This finding was supported by the results of the study of adult patients with depression who had survived a suicide attempt, and in whom NLR was also higher compared with controls [78]. The association between suicidal behavior in adults and high NLR values has been demonstrated not only in depression, but also in BD [26]. All these findings suggest age-related differences in the associations between IHRs and suicidal behavior in adolescents (increased NLR, MLR, and PLR [76]), young adults (only MLR increased [77]), and adult patients (only NLR increased [26, 61, 78]). It is noteworthy that non-suicidal self-injury in adolescents was associated with elevated MLR and PLR, but not NLR [54]. The differences between age groups in the correlation between certain IHRs with self-harm/suicidal behaviors may reflect age-related differences (to date unproven) in the biological mechanisms of such behaviors.
In our opinion, the associations between IHRs and certain clinical features of mental disorders (severity of symptoms, phase of the disease, presence of self-harm/suicidal behaviors) might hypothetically indicate a higher degree of activation of systemic inflammation in more severe/acute cases. One can assume that patients with higher IHR values (i.e. with more pronounced systemic inflammation) may represent a specific subtype of psychiatric disorders, likely differing in course and prognosis [16, 17]. However, the studies included in our review do not allow one to speculate on a causal relationship between IHRs and the severity of mental disorders. Elevated IHRs in various mental disorders may indicate common etiopathogenetic pathways, specifically common predisposing genetic factors [79–81]. Conversely, an increase in IHRs may be a consequence of a mental disorder, reflecting concomitant nonspecific physiological stress [17, 82]. It is quite likely that there is a bidirectional relationship between systemic inflammation and mental disorders, with each exerting a negative influence on the other [83]. Additionally, high intra- and inter-individual variability of inflammatory biomarkers is obvious, depending on a large number of factors (hereditary and environmental), which largely accounts for the low reproducibility and frequent inconsistency of study results [84].
Association between IHRs and the treatment response
The hypothetical influence of systemic inflammation on the development of treatment resistance [18, 19, 85] provides a rationale to study the relationship between IHRs and the response to treatment. We were unable to find studies that examined this relationship in adolescents with mental disorders. Studies in other age groups (young adults, adults) demonstrate conflicting results. On the one hand, higher values of the SII index and SIRI (systemic inflammatory response index) have been demonstrated in non-responders compared with responders in bipolar depression [86, 87]. On the other hand, elevated IHRs have been shown to be associated with higher treatment efficacy in psychotic depression [88, 89] and schizophrenia [90, 91].
Association between IHRs and metabolic disturbances
Our search strategy did not identify studies specifically aimed at assessing the relationship between IHRs and the metabolic syndrome or its components in adolescents with mental disorders. In most of the selected studies, excess weight or obesity was an exclusion criterium, which likely explains the lack of association between the body mass index and IHRs in the only study that assessed their relationship [52]. This assumption is supported by the results of the study in a sample of young adults (18–24 years) demonstrating higher NLR in MDD comorbid with obesity than in MDD without obesity, as well as a weak positive correlation between NLR and the body mass index [92].
Diagnostic and prognostic value of IHRs in adolescents with mental disorders
The findings from the studies showing higher IHRs in adolescents with mental disorders compared with controls [47–49, 51, 56] are promising in regards of using IHRs as diagnostic biomarkers. However, there is no consistent data on differences in IHRs in various diseases, which could have objectified and significantly facilitated the differential diagnosis, which poses particular difficulties in adolescents due to the transdiagnostic clinical presentations [42–44]. The results of IHR comparisons between various mental disorders in adults have low reproducibility. As an example, one study demonstrated that adults with exacerbation of schizophrenia had higher NLR than patients with BD in a manic episode [93], while the other study showed the opposite results [94]. In another study differences in IHRs between adult patients with bipolar and unipolar depression were observed [95], however in the large-scale cross-sectional study (13,888 participants) no significant differences in IHRs either between BD and MDD, or between BD and schizophrenia, were found [82].
Although the results of 2 studies included in our review indicate an association between IHRs and self-harm/suicidal behaviors [53, 54], the retrospective cross-sectional design of both studies excludes the possibility of using calculated cut-off values to predict the risk of future suicide attempts or non-suicidal self-injury. In the absence of studies linking IHRs to treatment response and metabolic syndrome, one can speculate on a possible use of IHRs for predicting the treatment response or assessing metabolic risks in adolescents solely on the grounds of the studies conducted in young adults and adults [88–92].
Perspectives for future research
One of the potential directions for future research would appear to be clarifying the role of systemic inflammation in the etiopathogenesis of mental disorders at different stages of their development, which requires a comprehensive assessment of not only IHRs, but also other immune inflammatory markers in conjunction with neurobiological, genetic, socio-demographic, and clinical variables across various age groups (younger children, adolescents, young adults, adults) at different stages of development/manifestation of a mental disorder.
Another direction is examining the diagnostic utility of IHRs, taking into account the transdiagnostic nature of clinical presentation in adolescence and complicated differential diagnosis. This area requires large-scale comparative studies, including samples of adolescents with various psychiatric diagnoses.
Evaluating IHRs as prognostic biomarkers also seems to be a promising direction. Models predicting the risks of suicide attempts and non-suicidal self-injury could assist in identifying adolescents at increased risk of self-harm/suicide and developing personalized preventive programs. Research on the prognostic value of IHRs in predicting treatment response and the risk of treatment resistance is essential for the development of adolescent-specific interventions aimed at the management of treatment resistance. Finally, there is an obvious research gap in the study of the relationship between IHRs and the metabolic syndrome, which are more prevalent in individuals with mental disorders than in the general population [96, 97]. The metabolic syndrome increases the risk of cardiovascular diseases [98, 99], leading to excessive early mortality and significant reduction in life expectancy for patients with mental disorders [100, 101]. That is why identifying adolescents with a high metabolic risk is, in our opinion, of great importance due to the potential reversibility of metabolic disturbances in the early stages. For the development of prognostic models predicting treatment response, the risk of self-harm/suicidal behaviors, and the risk of developing the metabolic syndrome, prospective studies are required.
CONCLUSION
The results of this scoping review support the hypothesis of systemic inflammatory mechanisms activation in mental disorders and demonstrate that IHRs can be used as indicators of immune inflammation in adolescent patients. Elevated IHRs have been observed across a wide range of mental disorders in adolescents (depression, psychotic disorders, OCD, ADHD, substance use disorders); however, the cut-off values for any of these disorders have not been calculated, which makes it impossible to assess IHRs diagnostic value. Also, there is no evidence to suggest that the association between IHRs and these disorders depend on age: similar patterns are observed in adolescents and adults. In both adolescents and adults, higher IHRs correspond to more severe/acute manifestations of mental disorders. Additionally, there is some evidence of age-specificity in the relationship of IHRs with both the severity of psychopathological symptoms and self-harm/suicidal behavior. At the same time, the limitations of the studies included in our review do not allow neither the assessment of the utility of IHRs as prognostic biomarkers for self-harm/suicidal behaviors in adolescents nor age-related comparisons. Assessment of the clinical value of IHRs as diagnostic and prognostic biomarkers requires confirmation of the reproducibility and specificity of their changes in various mental disorders in studies of higher methodological quality.