INTRODUCTION
Studies have shown that advanced age is one of the greatest risk factors for higher severity and worse outcome of COVID-19 [1–4] with neurological and psychiatric symptoms affecting 33–62% of patients within six months of recovery [5]. Some researchers suggest that SARS-CoV-2 may have an ability to invade the brain via the olfactory tract, circumventricular organs, leaky blood-brain barrier due to inflammation or direct damage of brain vascular endothelium or with migrating immune cells [6].
If specific routes of SARS-CoV-2 to enter the brain do exist than it may be associated with the distinct patterns of brain morphology changes. A recent article systematically reviewed brain imaging case series, case-control and cohort studies in patients with COVID-19 and found that alterations associated with COVID-19 predominated in the olfactory brain network, limbic and prefrontal structures [7]. About half of these studies used only visual evaluation of MRI scans, and among studies that used image statistical processing approach there were none that used matched control of age, gender and comorbidity (hypertension and/or diabetes mellitus type 2) in brain morphology analysis in older population affected and non-affected by COVID-19. Moreover, none of these studies evaluated brain changes specifically in older (65+ years old) population. Therefore, case-control study of MRI scans using image statistical processing approach and matched case-control analysis of elderly individuals with and without COVID-19 history is relevant.
Since October 2020 a longitudinal cohort study of patients with mild cognitive impairment (MCI) compared to healthy control was initiated in Psychiatric clinical hospital No 1 (Moscow, Russia) to identify unmet needs of MCI patients during COVID-19 pandemic. One of the aims of this study is a MRI-morphometry of brain scans in patients with mild cognitive impairment (MCI) and healthy control with and without reported COVID-19 history.
Our research question was: are there specific brain alterations in older people with reported history of COVID-19 infection compared to those without COVID-19 history?
METHODS
Study population
This study is a part of multidisciplinary project ‘Impact of the COVID-19 pandemic on the mental health of the elderly’ and is supported with grant of RFFI 20-04-60546. Individuals over 65 years old were eligible in the study. The study participants were selected among those who attended the Memory Clinic to treat cognitive impairment or at the outpatient unit of Moscow outpatient clinic No 152 (both are the branches of the Mental-Health Clinic No. 1 named after N.A. Alexeev) to treat somatic disorders other than acute or severe chronic somatic or infectious disease. Individuals with contraindications to MRI, with a history of dementia, Parkinson’s disease, Huntington’s disease, psychotic or other severe psychiatric disorders known to affect cognitive functioning, mood and anxiety disorders with onset before 45 years old, exacerbations or severe forms of chronic somatic diseases were not included. Also, people taking drugs with known negative or positive effect on cognitive functions were not allowed to participate in the study (see Supplements for a full list of exclusion criteria). The COVID-19 history was determined by the self-report and COVID-19 certificate. Current COVID-19 status was not checked with polymerase chain reaction rest (PCR) and none of participants were vaccinated as the study period ended before national vaccination campaign had been initiated.
Study design
This one-year study has longitudinal observational cohort design. Enrollment of subjects was performed 4th October 2020 to 30th April 2021. All participants underwent clinical examination by a psychiatrist and collection of medical history using checklist designed specifically for this study, including a checklist of individually significant neuropsychiatric and cognitive symptoms. Cognitive performance was assessed using Mini-mental state examination scale (MMSE) [8] and Montreal cognitive assessment scale (MoCA) [9]. After examination MRI-scanning was performed.
MRI scanning and Image processing
A MRI scanner (Toshiba, 1.5 Tl) at the Mental-health Clinic No1 Named After N.A. Alexeev was used to obtain structural MRI scans. The structural study was performed using a Sg 3d T1-weighted sequence (TR=12 ms, TE=5 ms, 200 sagittal slices, FOV 256 mm, FA 180, TI=300 ms, voxel size 1x1x1 mm3, average 2).
Image processing and segmentation were performed using Freesurfer v6.0 software package and morphological indices (thickness and volume of gray matter, volume of white matter, gyrification index, volume of gray matter by subcortical structures, etc.) were derived. Regional brain volumes (gray matter and white matter volumes) were selected for the purpose of this work.
FreeView imager (v7.1.0) was used to visually assess segmentation quality. Reproducibility of results was ensured by means of quality control of structural MRI (T1) images based on mriqc package and Image Quality Metrics (IQM) [10].
All calculations were performed on a cluster at Skoltech (Skolkovo Institute of Science and Technology), consisting of two computing units of the following configuration each: CPU: Intel Xeon 6 cores; RAM 64 GB; Storage: 1 TB GPU: 3 NVIDIA GeForce GTX 1080 Ti with 3584 Cuda cores, Memory capacity 11 GB, frequency 1500 MHz.
Statistical processing
Database was extracted on 9 September 2021. The primary endpoint was finding the differences in MRI volumes between those who experienced COVID-19 (COVID+) and those who had not (COVID-) in a whole study population. Mann-Whitney test was used to compare continuous variables while participant’s distribution by the categorical variables between groups was performed using Fisher exact test. All regional brain volumes were n-1 normalized to standardize the variables using the unbiased standard deviation. Group profiles of brain volumes means and medians of received z-scores were than additionally visually analyzed using parallel coordinates plots. In all statistical tests two-tails p <0.05 considered as statistically significant. We didn’t use multiple p correction because it would preclude finding of any significant differences given large number of regions (109 regions) to compare highly variable volumes.
To compare regional brain volumes, we also used case-control approach in subpopulations of COVID+ and COVID- groups matched by age, gender and history of hypertension and type II diabetes. The following strategy was used to search for matched subjects: exact matches on gender, hypertension stage and type II diabetes and fuzzy search for age within 2 years range.
Statistical processing was performed using Addinsoft (2022), XLSTAT statistical and data analysis solution. New York, USA. https://www.xlstat.com/en.
Ethics
The study was conducted according to Helsinki declaration and Good Clinical Practice (GCP) principles. It was approved by the local Ethical Committee of State Budgetary Institution of Health Care “Research Clinical Institute of Otorhinolaryngology named after L.I. Sverzhevsky” of the Department of Health of the City of Moscow (Protocol №5 from 20.09.20). All study participants signed informed consent.
RESULTS
Study population
Overall, 207 participants had processed Visit 1 MRI scan information in the study database as of 9th September 2021. Among them 24 indicated that they had a history of COVID-19 (either outpatient or hospitalization) before Visit 1 of the study (before October, 2020), with 4 of 24 (16.7%) were hospitalized with COVID-19. Thus, 24 participants were categorized in COVID+ group and n=183 in COVID- group. Their socio-demographic and medical characteristics are summarized in the Table 1.
Table 1. Clinical and socio-demographic characteristics of study population
|
Valid n |
COVID- (n=183) |
COVID+ (n=24) |
Test Statistic |
|
|
Age, median, 1st and 3rd quantile |
207 |
71 (66, 77) |
71 (68.4, 77.0) |
U=1973, p=0.418* |
|
Gender: Male, n (%) |
207 |
33 (18.0%) |
3 (12.5%) |
p=0.774** |
|
Education, n (%) |
205 |
p=0.539** |
||
|
College |
52 (28.7%) |
8 (33.3%) |
||
|
School |
15 (8.3%) |
3 (12.5%) |
||
|
University |
114 (63.0%) |
13 (54.2%) |
||
|
Work type in the life: Intellectual, n (%) |
200 |
144 (81.8%) |
21 (87.5%) |
p=0.774** |
|
Still working, n (%) |
200 |
6 (3.4%) |
2 (8.3%) |
p=0.247** |
|
Family: Yes, n (%) |
203 |
125 (69.8%) |
18 (75.0%) |
p=0.812** |
|
Self-efficient: Yes, n (%) |
202 |
135 (75.8%) |
18 (75.0%) |
p=1.000** |
|
Hobby: Yes, n (%) |
207 |
132 (72.1%) |
18 (75.0%) |
p=1.000** |
|
Somatic health |
||||
|
Any chronic disease: Yes, n (%) |
207 |
166 (90.7%) |
22 (91.7%) |
p=1.000** |
|
Diabetes type II: Yes, n (%) |
204 |
24 (13.3%) |
5 (20.8%) |
p=0.350** |
|
Hypertension, n (%) |
207 |
p=0.459** |
||
|
1 stage |
46 (25.1%) |
5 (20.8%) |
||
|
2 stage |
58 (31.7%) |
9 (37.5%) |
||
|
3 stage |
16 (8.7%) |
4 (16.7%) |
||
|
No |
63 (34.4%) |
6 (25.0%) |
||
|
Ischemic heart disease: Yes, n (%) |
204 |
51 (28.3%) |
7 (29.2%) |
p=1.000** |
|
Myocardial infarction history: Yes, n (%) |
207 |
6 (3.3%) |
1 (4.2%) |
p= 0.584** |
|
Oncology history: Yes, n (%) |
204 |
27 (15.0%) |
7 (29.2%) |
p=0.087** |
|
Obesity: Yes, n (%) |
204 |
38 (21.1%) |
4 (16.7%) |
p=0.790** |
|
Takes any antihypertensive drug: Yes, n (%) |
204 |
97 (53.9%) |
14 (58.3%) |
p=0.828** |
|
Takes any antidiabetic drug: Yes, n (%) |
204 |
7 (3.9%) |
2 (8.3%) |
p=0.286** |
|
Takes any anticoagulant: Yes, n (%) |
204 |
14 (7.8%) |
1 (4.2%) |
p=1.000** |
|
Takes aspirin: Yes, n (%) |
204 |
44 (24.4%) |
10 (41.7%) |
p=0.086** |
|
Mental health |
||||
|
MMSE total score, median, 1st and 3rd quantile |
204 |
27.0 (26.0, 28.0) |
28.0 (27.0, 29.0) |
U=1719, p=0.099* |
|
MoCA total score, median, 1st and 3rd quantile |
204 |
24.0 (21.0, 26.0) |
25.0 (22.4, 27.0) |
U=1789, p=0.170* |
|
Any affective disorder history: Yes, n (%)*** |
204 |
23 (12.8%) |
4 (16.7%) |
p=0.533** |
|
Any anxiety disorder history: Yes, n (%)*** |
204 |
5 (2.8%) |
3 (12.5%) |
p=0.054** |
|
OCD: Yes, n (%)*** |
204 |
1 (0.6%) |
0 (0.0%) |
p=1.000** |
|
Significant symptoms |
||||
|
Apathy: Yes, n (%) |
203 |
16 (8.9%) |
4 (16.7%) |
p=0.267** |
|
Headache: yes, n (%) |
203 |
47 (26.3%) |
1 (4.2%) |
p=0.019** |
|
Vertigo, dizziness: Yes, n (%) |
203 |
39 (21.8%) |
5 (20.8%) |
p=1.000** |
|
Sleep: Yes, n (%) |
203 |
89 (49.7%) |
13 (54.2%) |
p=0.828** |
|
Poor attention, concentration: Yes, n (%) |
203 |
41 (22.9%) |
7 (29.2%) |
p=0.609** |
|
Inaccurate movements: Yes, n (%) |
203 |
15 (8.4%) |
1 (4.2%) |
p=0.699** |
|
Fatigue, retardation: Yes, n (%) |
203 |
55 (30.7%) |
9 (37.5%) |
p=0.492** |
|
Hypothymia: Yes, n (%) |
203 |
26 (14.5%) |
2 (8.3%) |
p=0.541** |
|
Gastro-intestinal: Yes, n (%) |
203 |
38 (21.2%) |
3 (12.5%) |
p=0.423** |
|
Irritability: Yes, n (%) |
203 |
37 (20.7%) |
8 (33.3%) |
p=0.190** |
|
Affective liability: Yes, n (%) |
203 |
45 (25.1%) |
5 (20.8%) |
p=0.803** |
|
Heart palpitations: Yes, n (%) |
203 |
26 (14.5%) |
6 (25.0%) |
p=0.229** |
|
Weakness in legs: Yes, n (%) |
203 |
39 (21.8%) |
6 (25.0%) |
p=0.794** |
|
Anxiety: Yes, n (%) |
203 |
38 (21.2%) |
5 (20.8%) |
p=1.000** |
|
Spatial orientation: Yes, n (%) |
203 |
20 (11.1%) |
2 (8.3%) |
p=1.000** |
|
Memory fixation: Yes, n (%) |
203 |
101 (56.4%) |
15 (62.5%) |
p=0.663** |
|
Calculation: Yes, n (%) |
203 |
44 (24.6%) |
5 (20.8%) |
p=0.804** |
|
Tinnitus: Yes, n (%) |
203 |
44 (24.6%) |
8 (33.3%) |
p=0.454** |
Note: Valid n — number of non-missing value. * — Mann-Whitney test. ** — Fisher exact test. *** — With onset after 45-year-old.
Matching for the age (fuzzy matching ±2 years), gender (exact match), hypertension stage (exact match) and history of type II diabetes (exact match) revealed 22 matched pairs of participants leaving 2 COVID+ participants without match. These characteristics are depicted in the Table 2.
Table 2. Clinical and socio-demographic characteristics of study subpopulations matched by age, gender, hypertension stage and type II diabetes history
|
Valid n |
COVID- (n=22) |
COVID+ (n=22) |
Test Statistic |
|
|
Age, median, 1st and 3rd quantile |
44 |
71,5 (68; 75,8) |
71 (68,3; 77) |
U=238,5, p=0,953* |
|
Gender: Male, n (%) |
44 |
2 (9,10%) |
2 (9,10%) |
p=1,000** |
|
Education, n (%) |
44 |
p=0,555** |
||
|
College |
5 (22,70%) |
8 (36,40%) |
||
|
School |
2 (9,10%) |
3 (13,60%) |
||
|
University |
15 (68,20%) |
11 (50,00%) |
||
|
Work type in the life: Intellectual, n (%) |
44 |
17 (81,00%) |
19 (86,40%) |
p=0,698** |
|
Still working, n (%) |
44 |
1 (4,50%) |
1 (4,50%) |
p=1,000** |
|
Family: Yes, n (%) |
44 |
11 (50,00%) |
17 (77,30%) |
p=0,116** |
|
Self-efficient: Yes, n (%) |
44 |
18 (81,80%) |
16 (72,70%) |
p=0,721** |
|
Hobby: Yes, n (%) |
44 |
132 (72.1%) |
18 (75.0%) |
p=1.000** |
|
Somatic health |
||||
|
Any chronic disease: Yes, n (%) |
44 |
22 (100,00%) |
20 (90,90%) |
p=0,488** |
|
Diabetes type II: Yes, n (%) |
44 |
3 (13,60%) |
3 (13,60%) |
p=1,000** |
|
Hypertension, n (%) |
44 |
p=1,000** |
||
|
1 stage |
4 (18,20%) |
4 (18,20%) |
||
|
2 stage |
9 (40,90%) |
9 (40,90%) |
||
|
3 stage |
3 (13,60%) |
3 (13,60%) |
||
|
No |
6 (27,30%) |
6 (27,30%) |
||
|
Ischemic heart disease: Yes, n (%) |
44 |
4 (18,20%) |
5 (22,70%) |
p=1,000** |
|
Myocardial infarction history: Yes, n (%) |
44 |
0 (0,00%) |
1 (4,50%) |
p=1,000** |
|
Oncology history: Yes, n (%) |
44 |
6 (27,30%) |
6 (27,30%) |
p=1,000** |
|
Obesity: Yes, n (%) |
44 |
5 (22,70%) |
4 (18,20%) |
p=1,000** |
|
Takes any antihypertensive drug: Yes, n (%) |
44 |
14 (63,60%) |
12 (54,50%) |
p=0,760** |
|
Takes any antidiabetic drug: Yes, n (%) |
44 |
0 (0,00%) |
1 (4,50%) |
p=1,000** |
|
Takes any anticoagulant: Yes, n (%) |
44 |
2 (9,10%) |
1 (4,50%) |
p=1,000** |
|
Takes aspirin: Yes, n (%) |
44 |
6 (27,30%) |
8 (36,40%) |
p=0,747** |
|
Mental health |
||||
|
MMSE total score, median, 1st and 3rd quantile |
44 |
27 (26; 28) |
27,5 (27; 29) |
U=183,5, p=0,170* |
|
MoCA total score, median, 1st and 3rd quantile |
44 |
25 (21; 27) |
25 (23; 27) |
U=221, p=0,843* |
|
Any affective disorder history: Yes, n (%) |
44 |
2 (9,10%) |
3 (13,60%) |
p=1,000** |
|
Any anxiety disorder history: Yes, n (%) |
44 |
0 (0%) |
3 (13,60%) |
p=0,233** |
|
OCD: Yes, n (%) |
44 |
0 |
0 |
|
|
Significant symptoms |
||||
|
Apathy: Yes, n (%) |
44 |
2 (9,10%) |
4 (18,20%) |
p=0,664** |
|
Headache: yes, n (%) |
44 |
9 (40,90%) |
1 (4,50%) |
p=0,009** |
|
Vertigo, dizziness: Yes, n (%) |
44 |
8 (36,40%) |
5 (22,70%) |
p=0,510** |
|
Sleep: Yes, n (%) |
44 |
13 (59,10%) |
12 (54,50%) |
p=1,000** |
|
Poor attention, concentration: Yes, n (%) |
44 |
3 (13,60%) |
7 (31,80%) |
p=0,281** |
|
Inaccurate movements: Yes, n (%) |
44 |
1 (4,50%) |
1 (4,50%) |
p=1,000** |
|
Fatigue, retardation: Yes, n (%) |
44 |
5 (22,70%) |
9 (40,90%) |
p=0,332** |
|
Hypothymia: Yes, n (%) |
44 |
4 (18,20%) |
2 (9,10%) |
p=0,664** |
|
Gastro-intestinal: Yes, n (%) |
44 |
5 (22,70%) |
3 (13,60%) |
p=0,698** |
|
Irritability: Yes, n (%) |
44 |
6 (27,30%) |
7 (31,80%) |
p=1,000** |
|
Affective liability: Yes, n (%) |
44 |
6 (27,30%) |
5 (22,70%) |
p=1,000** |
|
Heart palpitations: Yes, n (%) |
44 |
3 (13,60%) |
5 (22,70%) |
p=0,698** |
|
Weakness in legs: Yes, n (%) |
44 |
5 (22,70%) |
5 (22,70%) |
p=1,000** |
|
Anxiety: Yes, n (%) |
44 |
6 (27,30%) |
5 (22,70%) |
p=1,000** |
|
Spatial orientation: Yes, n (%) |
44 |
2 (9,10%) |
2 (9,10%) |
p=1,000** |
|
Memory fixation: Yes, n (%) |
44 |
13 (59,10%) |
15 (68,20%) |
p=0,755** |
|
Calculation: Yes, n (%) |
44 |
4 (18,20%) |
4 (18,20%) |
p=1,000** |
|
Tinnitus: Yes, n (%) |
44 |
5 (22,70%) |
6 (27,30%) |
p=1,000** |
Note: Valid n — number of non-missing value. * — Mann-Whitney test. ** — Fisher exact test.
In both instances of comparison between COVID+ and COVID- subjects (Tables 1 and 2) there were no significant differences on most of variables. Most of the participants were women, retired, with the background of higher education. More than two thirds of them lived with their families and described themselves as self-efficient in most life areas. Somatic disorders had generally equal distribution between groups and prevalent (more than 90% had any chronic medical condition). Mental disorders and individually significant symptoms report (yes / no in a specific checklist, Table 1 and 2) were equally distributed except headaches (significantly more frequent in the COVID- group). Cognitive status (total scores on MMSE and MoCA) was similar in both groups.
Both MMSE and MoCA total scores in all subjects (n=207) showed a significant weak correlation with total brain volume (Spearman R=0.23, p=0.001 and Spearman R=0.20, p=0.004, respectively) and cerebrospinal fluid (CSF) total volume (Spearman R=-0.23, p <0.001 and Spearman R=-0.27, p <0.001, respectively).
Regional brain volumes in COVID+ and COVID- groups
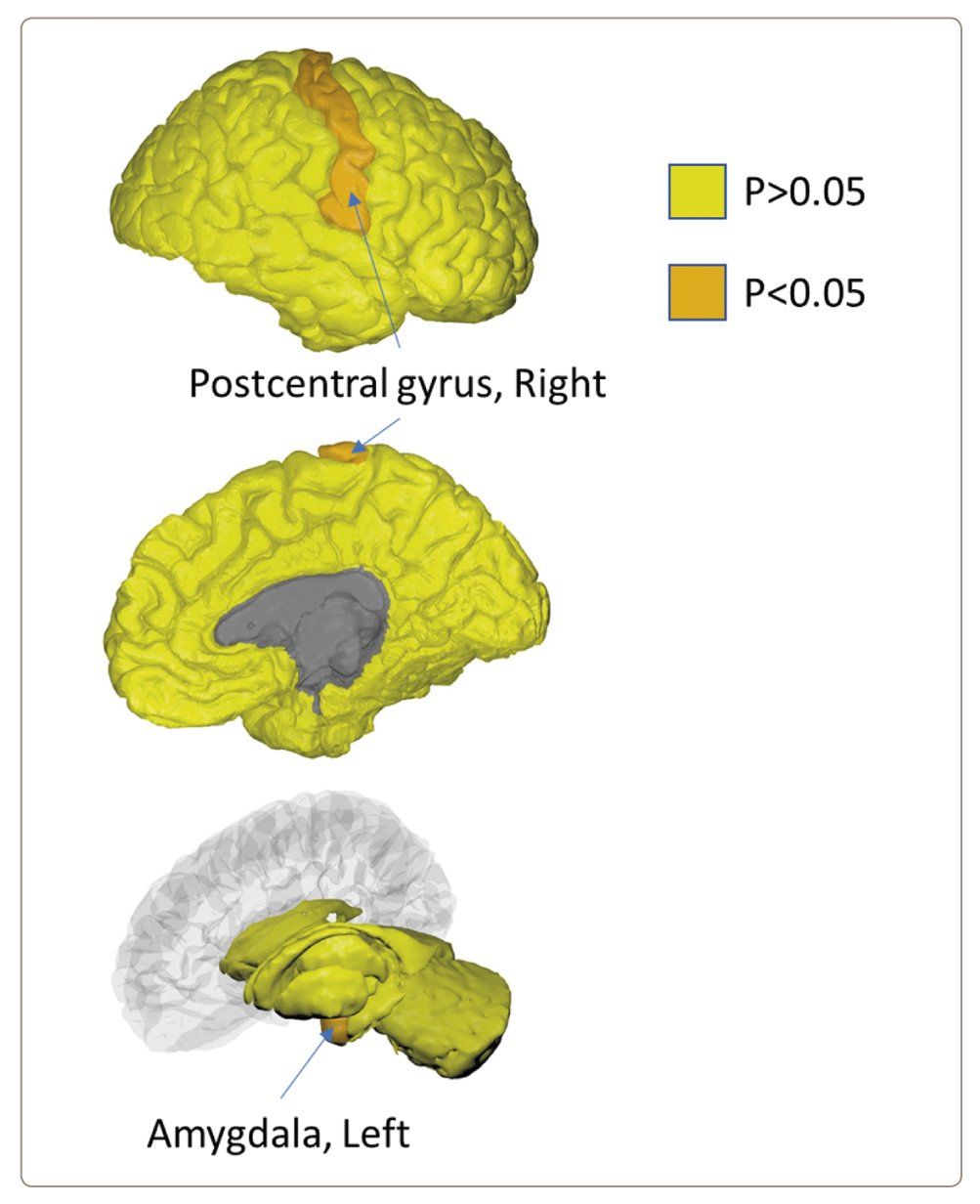
Comparison of brain regional volumes in whole study population (n=207) revealed differences only in two regions: right postcentral gyrus (median 8055.5 mm3 in COVID+ vs. 8434.0 mm3 in COVID-, U=1821.5, p=0.045, Mann-Whitney test) and left amygdala (median 1199.3 mm3 in COVID+ vs. 1263.7 mm3 in COVID-, U=1839.0, p=0.044, Mann-Whitney test) (Figure 1).
Figure 1. Significant differences between COVID+ and COVID- groups on regional brain volumes (Mann-Whitney test) in whole study population (n=207).
Detailed statistical results can be found in Supplementary 1.
Though, comparison of matched (age, gender and medical conditions) subpopulations (n=44) did not find any significant differences in regional brain volumes (Supplementary 2).
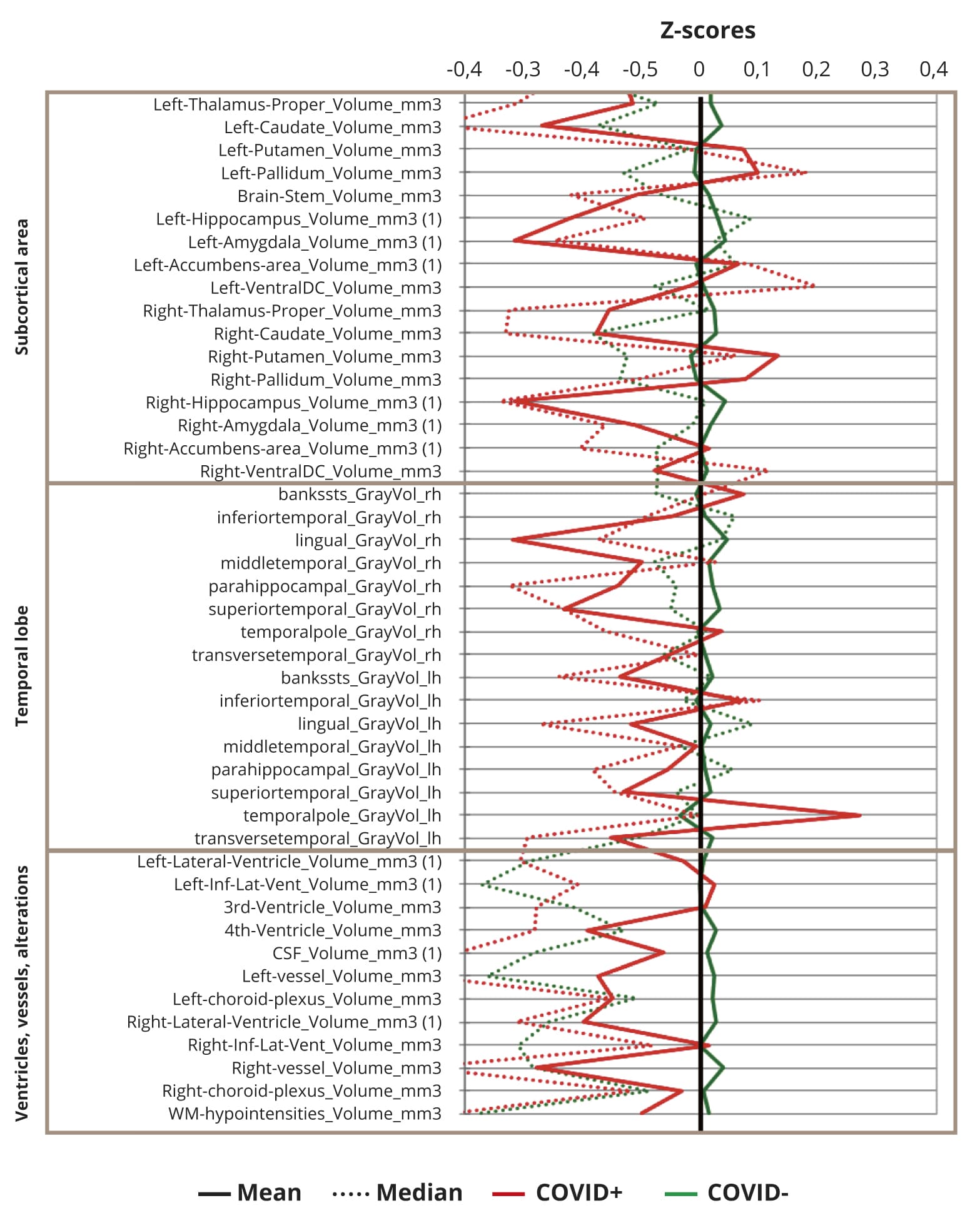
Parallel coordinate plots of normalized (n-1) MRI regional volumes are depicted on the Figure 2.
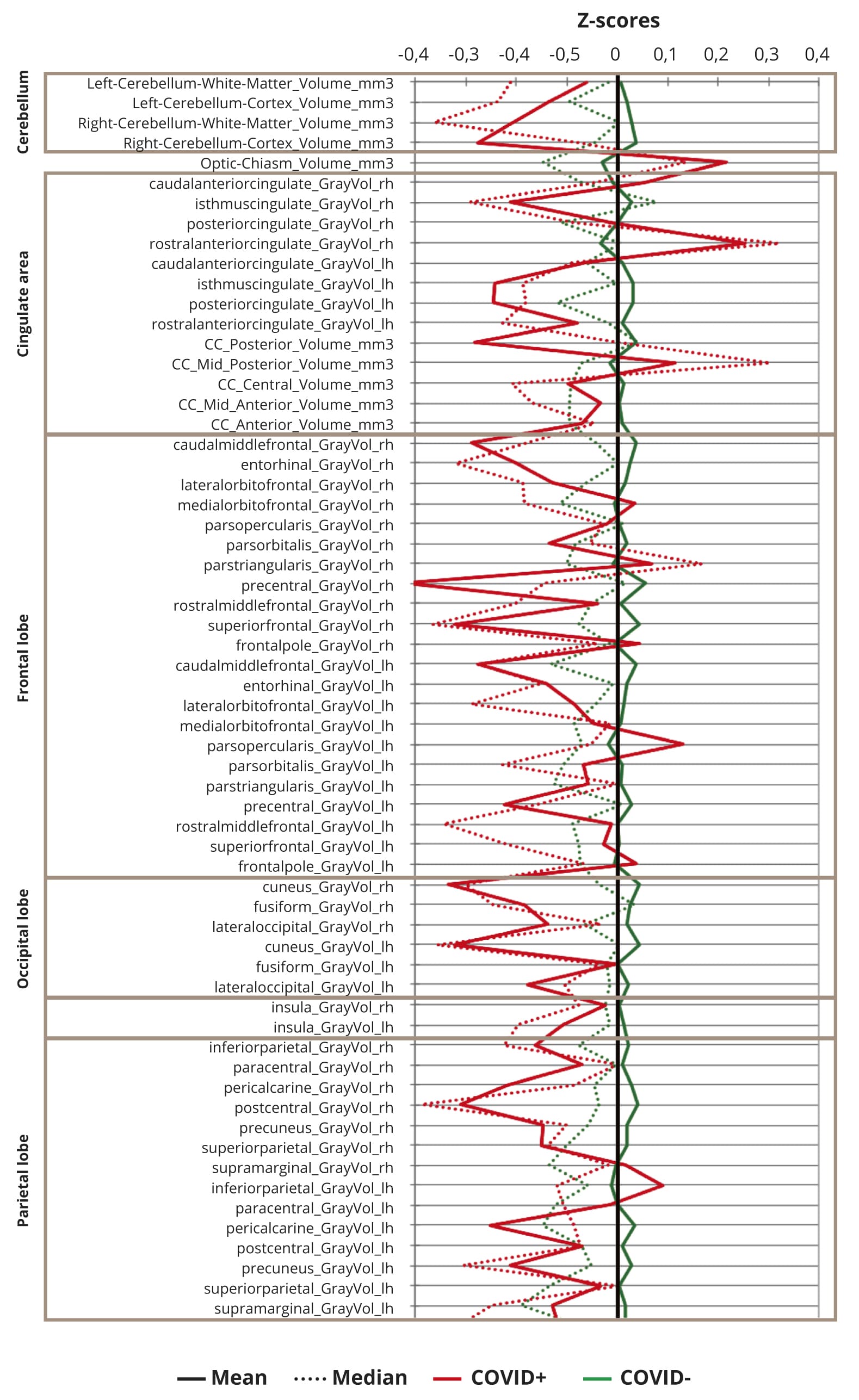
Figure 2. Standardized brain volumes profiles in COVID+ and COVID- participants (n=207).
Note: All means (solid line) or medians (dashed line) of residuals of regional brain volumes in both COVID+ (red) and COVID- (green) participants were within 1 standard deviation from mean values.
None of means or medians of z-scores exceed 1, reflecting that there were no differences in regional brain volumes larger than one standard deviation of study population means, though, generally most of z-scores are slightly decreased in COVID+ against COVID- subjects.
DISCUSSION
Our study revealed differences in regional brain volumes between COVID+ and COVID- groups: right postcentral gyrus gray matter and left amygdala volumes found to be significantly lower in older people who had a history of COVID-19. These differences were not supported with any differences in reporting of personally meaningful neurocognitive and neuropsychiatric symptoms, including anxiety and somatic complaints with except of headaches that were more prevalent in COVID- group.
Olfactory tract projections are considered as a possible gateway of SARS-CoV-2 invasion into the brain. Amygdala is a part of limbic system that receives projections from olfactory bulbs [11, 12]. According to recent research [13] a comparison of MRI scans before and after COVID-19 found that patients after COVID-19 had greater grey matter loss in the central nucleus of the amygdala than those who had no history of COVID-19. Another study [14] found hypometabolism in the right temporal lobe, including amygdala in patients with long COVID-19. Though, in these studies changes in amygdala were accompanied with alterations in other brain regions, including central olfactory complex (piriform cortex, enthorhinal cortex) and secondary olfactory areas (hippocampus, thalamus, orbitofrontal cortex) [7, 13, 14].
Contrary, in our study we did not find significant morphological changes in other brain regions. One possible explanation is that our study included population who experienced mild forms of COVID-19 (only 4 of 24 reported that they were hospitalized) that did not associate with brain tissue lesions. This explanation is supported by the fact that we did not find bilateral morphological changes that can be expected from olfactory route of virus penetration through blood-brain barrier. Also, this may result from a selection bias while enrolling patients into the study, those who had more severe forms of COVID-19 may not had applied for treatment to Memory clinic due to either restriction on transit for people older than 65 years or precaution/incapacity to move across the city.
Many MRI studies of brain structural changes in patients experienced COVID-19 reported alteration in different brain areas other than olfactory system [7]. Nevertheless, most of these studies did not include control sample and studied patients with COVID-19 severe enough to be hospitalized. The recent brain imaging study before and after COVID-19 included patients with second MRI scan after 35–407 days after recovery from in most cases mild forms of the disease in comparison to healthy control [13]. This study revealed decrease of cortical thickness in the lateral orbitofrontal cortex, generally greater brain size reduction, increase in diffusion indices and CSF volume. Though, a comparison of hospitalized cases with either non-hospitalized or control subgroups failed to detect marked differences due to decrease in the sample size. While this study that was conducted on a population that was very close to our study revealed significant differences in brain morphology, it didn’t account control for comorbid disorders like hypertension and type II diabetes that also associated with brain structure changes. Moreover, these comorbid disorders are known to be associated with increased risk of COVID-19 complications [15, 16]. Thus, underestimation of these medical conditions may lead to bias in the results.
In our study we attempted to control these conditions. When compared subpopulations matched on gender, age and comorbid medical condition (hypertension and/or type II diabetes) regional brain volume differences disappeared. This may be due to that controlled conditions account for more of the variability in regional brain volumes than COVID-19 history. The median MMSE score before matching was mathematically lower in the COVID- compared to COVID+ group and in opposite to our results one could expect that if COVD-19 is associated with brain leisure than the matching on MMSE score will make differences even larger. Nevertheless, as expected, total MMSE (and MoCA) scores in whole study population showed significant positive correlation with total grey matter volume and negative correlations with total CSF volume showing adequate association between structural brain alterations and cognitive functions. This may indicate that an alternative explanation can take place: the decrease in sample size reduces the statistical power to detect brain differences between groups.
Study limitations
One of the study limitations was that COVID-19 status was not confirmed in the laboratory at the study entry but only with history and COVID-19 certificate provided by the participants. Thus, it is possible that we misclassified those participants who recovered from COVID-19 but is not aware about that. Only four subjects in our study had COVID-19 severity enough to be hospitalized, thus the rest 20 subjects possibly experienced only mild forms of a disease limiting generalizability of study results. There can be a selection bias originating from personal precautions and governmental restrictions on movement amid COVID-19 pandemic. Also, we did not analyze the time between COVID-19 and MRI scan though this time unlikely exceeded a 13-month period as by 31.03.2020 only 1836 cases of COVID-19 were totally registered in Russia. Finally, only 24 participants had a known reported history of COVID-19 thus this study may be underpowered to detect brain morphology changes.
CONCLUSION
We didn’t find definite associations of any regional brain volumes differences with COVID-19 history in people older than 65 years. Our study results are based on a population exposed to relatively mild forms of COVID-19. Thus, given study limitations, these results can’t be generalized to other people who recovered from COVID-19. Further better balanced and controlled and larger studies on an association of brain morphology with COVID-19 experience stratified by the severity in older people would help to disentangle relationships between COVID-19 severity and brain morphology changes.