Introduction
The existing research has largely focused on exploring how the duration of untreated illness (DUI) influences its further course. DUI is defined as the period between the onset of the first clinical symptoms of a disease and the beginning of adequate treatment [1, 2]. There is a large number of studies that have established how the duration of untreated psychosis (DUP) influences the effectiveness of therapy and the outcomes [3, 4]. A shorter DUP has shown correlation with better treatment outcomes [3], whereas a longer DUP has been associated with more severe, positive, and negative symptoms and lower chances of achieving remission [4]. Furthermore, a longer DUP has been associated with a more severe global psychopathology and poorer functioning during follow-up [4]. However, DUI in youth depression and its impact on the further course of the disease remains scarcely investigated. It has been found that a longer DUI negatively impacts the course of affective disorders [1, 2], reduces the duration of remission [2], and is associated with a higher risk of suicide [5]. According to other researchers, a longer DUI correlates with the severity of the cognitive impairments associated with depression [6].
It is often difficult to determine exactly when depression begins. According to researchers in the field, a major depressive episode is typically preceded by several non-affective symptoms, including dysthymic illness, cognitive disorders, episodes of apathy, decreased motivation, and obsession symptoms and irritability [7, 8]. The beginning of youth depression is frequently characterized by episodes of substance abuse and aggressive, self-harm behavior [9, 10]. These symptoms are often not associated with reduced premorbid functioning and, therefore, are not recognized as mental disorders. This leads to either refusal of medical care or referral to primary care, in place of qualified mental health care [11]. Yet, depression in youth may be the first symptom of a variety of non-affective disorders, including schizophrenia spectrum disorders such as schizotypal disorder and the prodromal stage of schizophrenia [12, 13]. It is clear that early identification of youth depression is crucial not only for the further course of depression, but also as part of the diagnosis and prevention against a wide spectrum of mental health issues.
Against this background, the present study aims to determine how the DUI affects the severity of symptoms during the first depressive episode in youths and the degree of symptom reduction after treatment, with an analysis of the comorbidity of non-psychotic mental disorders.
Methods
Study design
A cross-sectional study design was applied for the purposes of the current study. Cross-sectional design is often regarded as a method of choice when there is a need to collect data from different individuals at a single point in time. In the context of the current study, using this design was advantageous in several respects. Firstly, it allowed us to establish the average duration of the DUI by the time of the first hospitalization. Secondly, it made it possible to compare the severity of depressive symptoms in patients with different DUI. Thirdly, it enabled us to determine the effectiveness of treatment in patients recruited at the same time.
Sampling and recruitment
The convenience sampling strategy was used to select the participants in the study. All patients hospitalized with the first depression episode at the Department of Youth Psychiatry of the Mental Health Research Centre from April 1, 2021 to May 30, 2022 were invited to participate.
Participants were eligible if they met the following criteria: 1) categorized as young people and youths (15–29 years old), according to the classification of the World Health Organization [15]; 2) hospitalized with the first depressive episode; 3) diagnosed within a range of nonpsychotic mental disorders according to the International Classification of Diseases, version 2019 (ICD-10); and 4) showing no indication of previous adequate treatment based on the National Institute for Health and Care Excellence Guidelines [16] and clinical recommendations of the Russian Society of Psychiatrists [17]. Treatment was considered as inadequate if low doses of antidepressants were administered or the duration of the treatment was under six months without any clinical response.
Participants were excluded from the study if they met one of the following criteria: 1) diagnosed with psychotic disorders, 2) presence of clinically significant somatic and neurological diseases at the time of the study, and 3) refusal to participate in the study at any stage during hospitalization.
Procedure
Hospital medical form 003 U (a mandatory form for patients admitted for inpatient treatment) was used to collect socio-demographic and clinical data, including medical history, the age when the symptoms (apathy, irritability, decreased motivation and other negative symptoms) preceding the first depression episode appeared, duration of the current depressive symptoms, and details regarding previous antidepressant treatment.
For the purposes of this study, both affective and “prodromal” symptoms were assessed. The assessment was conducted twice: at the time of admission (the first assessment) and at the time of discharge (the second assessment).
Affective symptoms were evaluated using the Hamilton Depression Rating Scale (HDRS) [18]. It is a 21-item Likert questionnaire in which a total score is calculated as the sum of all individual items.
“Prodromal” symptoms, including attenuated positive, negative and disorganization symptoms, were assessed using the Scale of Prodromal Symptoms (SOPS) [19] and the Scale for Assessment of Negative Symptoms (SANS) [20]. SOPS is a Likert scale which contains four main sections assessing (P) Positive Symptoms (5 items), (N) Negative Symptoms (6 items), (D) Disorganized Symptoms (4 items), and (G) General Symptoms (4 items). Positive Symptoms are rated on a scale from 0 (Absent) to 6 (Severe and Psychotic). Negative, Disorganized and General Symptoms are rated from 0 (Absent) to 6 (Extreme). SANS is a 25-item Likert scale which consists of five domains, including Affective Flattening or Blunting, Alogia, Avolition — Apathy, Anhedonia — Asociality, and Attention. A set of different symptoms is rated within each domain from 0 (Absent) to 5 (Severe).
After collecting all the clinical and psychometric data, a possible comorbid diagnosis was verified. It is important to note that there were difficulties in confirming comorbid bipolar disorder, recurrent depressive disorder, and persistent mood (affective) disorders due to the young age of the participants and their hospitalization being the first one. Therefore, the provided comorbid diagnoses are rather tentative here.
Treatment effectiveness is defined as the difference between the adopted scales score at the time of admission and at the time of discharge converted into percentages.
In order to evaluate the influence of the side effects of the therapy on the daily performance, all participants were examined on the 48-item Likert UKU Side Effect Rating Scale [21].
Data analysis
Our statistical analysis was carried out using the StatSoft’s statistical analysis software package Statistica 12.0. Firstly, sociodemographic data was analysed using descriptive statistical methods. The data was presented in mean values (standard deviation) and reported as Mean ± (SD). Secondly, depressive symptoms were analysed using the HDRS and “prodromal” symptoms were analysed using the SOPS and the SANS. The data was presented in median values and quartile range and reported as Me [Q1; Q3]. The first quartile [Q1] is defined as the middle number between the minimum value and the median, whereas the third quartile [Q3] is the middle value between the median and the maximum value. The Mann–Whitney U test was used to compare the differences on these scales between two independent groups. Correlations between the DUI and severity of clinical symptoms at the time of admission and before discharge were defined using the Spearman’s rank correlation coefficient as a nonparametric measure of rank correlation.
Research governance
The study was in line with the Helsinki Declaration and was approved by the Local ethics committee of the Mental Health Research Centre (Protocol №746 of 18.03.2021). All patients signed an informed consent form. Clinical data was collected in compliance with Order of the Ministry of Health of the Russian Federation (from 13.12.2015) N 1034n “On approval of the procedure specialized medical care ‘Psychiatry-Narcology’ and dispensary procedure monitoring of persons with mental disabilities and (or) behavioral disorders associated with substance abuse” and the regulations of the Mental Health Research Centre.
Results
Sample characteristics
Overall, 52 male patients hospitalized with the first depressive episode were included in the study. At the time of admission, a severe depressive episode without psychotic symptoms (classified as F32.2 according to the ICD-10) was established in 88.5% (n=46) of the patients, whereas a moderate depressive episode, (classified as F32.1 according to the ICD-10), was determined in 11.5% (n=6) of the patients. The participants were treated with antidepressants and antipsychotics. In particular, 10 patients (19.2%) had received inadequate antidepressant therapy during the period of untreated illness. The average dose was 7.6±3.2 mg/day in fluoxetine equivalent, and the average duration was 26.5±14.7 days. Overall characteristics of the sample are presented in Table 1.
Table 1. Characteristics of the sample
|
Characteristic |
Patients, total (n=52) |
|
Sex (%) |
Male (100%) |
|
Age when depressive symptoms appeared, years |
16.1±3.6 |
|
Age of the first hospitalization, years |
19.2±2.1 |
|
Mean DUI, months |
35.9±17.0 |
|
Level of education |
|
|
Basic general education, n (%) |
14 (26.9) |
|
Secondary general education, n (%) |
35 (67.3) |
|
Higher education, n (%) |
3 (5.8) |
|
Occupation |
|
|
Student, n (%) |
29 (55.8) |
|
Full-time employment, n (%) |
6 (11.5) |
|
Part-time employment, n (%) |
8 (15.4) |
|
Non-Employment, n (%) |
9 (17.3) |
Assessment of all participants
At the time of admission (first assessment), the degree of depression among all participants based on HDRS was 32 [28; 35], indicating severe depression [22]. The total score on the SOPS was 50 [45; 55], which demonstrated the presence of “prodromal” positive, negative symptoms and symptoms of disorganization in the patient of the clinical group. The overall score on the SANS was 49 [42; 54.5], which supported previous results and revealed the presence of negative symptoms. During the second assessment (before discharge), the degree of depression based on HDRS was 10.5 [6.75; 14.25], which suggested mild depression [22]. The total score on the SOPS was 24.5 [18.75; 32], whereas the total score on the SANS was 27 [19; 35]. Although these values suggest a reduction in symptoms acuity, they cannot be perceived as a sign of complete remission.
Assessment of participants depending on the mean DUI value
Based on the mean DUI value, which was 35.8±17.0 months, the patients were divided into two groups: the first group, with a DUI of more than 36 months (59.6%, n=31), and the second group — DUI of less than 36 months (40.4%, n=21). The length of hospitalization was equal to the duration of active treatment and lasted 42.9±27.6 days in the whole sample. The average dose of received antidepressants in the fluoxetine equivalent in the first group was 32.2±25.0 mg/day; in the second group, 43.4±28.3 mg/day, and the average dose of antipsychotics in the chlorpromazine equivalent in the first group was 295.9±187.8 mg/day; and in the second group — 294.3±144.5 mg/day. There were no statistical differences between the groups in the fluoxetine equivalent (p=0.191; U=255.0) and in the chlorpromazine equivalent (p=0.787; U=310.0).
Cross-group comparison between participants with different DUI shows that the reduction of HDRS scores was significantly higher in the first group (p=0.019) at the time of discharge, with no differences in the severity of depressive symptoms (p=0.544) at the time of admission. No other statistically valid differences were found. Assessment of the severity of affective and “prodromal” symptoms in the two groups of patients with different DUI at the time of admission and before discharge are presented in Table 2.
Table 2. Severity of affective and “prodromal” symptoms in the two groups of patients at the time of admission and before discharge
|
Parameters |
1st group (DUI >36 months), n=21 |
2nd group (DUI <36 months), n=31 |
U, p-value |
|||
|
1st assessment |
2nd assessment |
1st assessment |
2nd assessment |
1st assessment |
2nd assessment |
|
|
HDRS total score [Q1;Q3] |
32 [28; 36.5]** |
12 [7.5; 18.5]* |
33 [28; 35] |
10 [4; 11] |
292.5, 0.544 |
199.5, 0.019 |
|
SOPS total score [Q1;Q3] |
51 [45; 54.5] |
28 [19; 35.5] |
48 [45; 55] |
21 [16; 29] |
308.5, 0.758 |
227.5, 0.069 |
|
SOPS positive score [Q1;Q3] |
8 [6; 12] |
3 [2.5; 6] |
8 [6; 12] |
2 [1; 4] |
313.0, 0.822 |
246.0, 0.140 |
|
SOPS negative score [Q1;Q3] |
20 [18; 22.5] |
13 [8; 15]° |
20 [18; 21] |
10 [6; 12] |
294.0, 0.563 |
227.5, 0.069 |
|
SOPS disorganization score [Q1;Q3] |
10 [8; 12] |
5 [4; 7]° |
8 [8;11] |
5 [3; 6] |
266.0, 0.271 |
234.5, 0.091 |
|
SOPS general score [Q1;Q3] |
12 [11; 13.5] |
5 [4; 7] |
12 [11; 14] |
5 [3; 7] |
299.5, 0.634 |
267.0, 0.279 |
|
SANS total score [Q1;Q3] |
51 [42.5; 56] |
32 [20.5; 37.5]° |
48 [42; 51] |
24 [17; 28] |
273.5, 0.337 |
235.0, 0.093 |
|
SANS “Affective Flattening or Blunting” score [Q1;Q3] |
16 [13; 19] |
10 [6; 13.5] |
15 [13; 18] |
8 [5; 12] |
275.5, 0.544 |
252.0, 0.173 |
|
SANS “Alogia” score [Q1;Q3] |
7 [6; 9] |
4 [2; 5]° |
6 [5; 8] |
2 [1; 3] |
252.0, 0.173 |
232.0, 0.082 |
|
SANS “Avolition – Apathy” score [Q1;Q3] |
9 [7; 10] |
5 [3.5; 7] |
9 [8; 0] |
5 [3; 6] |
311.0, 0.794 |
272.0, 0.323 |
|
SANS “Anhedonia – Asociality” score [Q1;Q3] |
12 [10; 13] |
8 [5; 9.5]° |
12 [11; 13] |
5 [4; 8] |
312.0, 0.808 |
223.5, 0.058 |
|
SANS “Attention” score [Q1;Q3] |
6 [5; 6] |
3 [2.5; 4]° |
6 [5; 7] |
3 [2; 4] |
304.0, 0.695 |
235.0, 0.093 |
Note: * — statistically significant (р <0.05); ° — trend towards statistical significance (0.05< р <0.1) between the first group and the second group; ** — the median value, values of Q1 and Q3 are given in square brackets.
Defining a possible comorbid diagnosis
Further, possible comorbid diagnoses according to the ICD-10 were verified in 67.3% (n=35) of patients while a single depressive episode (classified as F32 according to the ICD-10) remained a primary diagnosis in 16.1% (n=5) of patients in the first group and in 57.1% (n=12) in the second group (Table 3).
Table 3. Possible comorbid diagnoses in the two groups of patients
|
Comorbid diagnoses (ICD-10) |
1st group (DUI >36 months), n (%) |
2nd group (DUI <36 months), n (%) |
|
Schizotypal disorder (F21) |
11 (42.3) |
2 (22.2) |
|
Bipolar disorder (F31) |
3 (11.5) |
3 (33.3) |
|
Recurrent depressive disorder (F33) |
8 (30.8) |
1 (11.1) |
|
Persistent mood (affective) disorders (F34) |
2 (7.7) |
2 (22.2) |
|
Personality disorders (F60) |
2 (7.7) |
1 (11.1) |
|
Overall |
26 (83.9) |
9 (42.9) |
Treatment effectiveness
According to the clinical recommendations accepted in the Russian Federation, the therapy is considered to be sufficiently effective if the reduction of symptoms amounts to more than 50% and partly effective if the reduction of symptoms is less than 50%, but more than 30%. When comparing the results obtained during the first and second assessments (see Table 2), it was noted that the severity of affective symptoms based on the HDRS results had reduced by 62.5% in the first group and 69.7% in the second group. The severity of “prodromal” symptoms based on the SOPS results was down by 45.1% in the first group and 56.3% in the second group, whereas the severity of negative symptoms on the SANS had decreased by 37.2% and 50.0%, respectively. The highest treatment effectiveness was noticed in the reduction in positive symptoms based on the SOPS positive subscale by 62.5% in the first group and 75% in the second group. The lowest reduction in symptoms, in turn, was observed in negative symptoms on the SOPS negative subscale (35%), SANS “Affective Flattening or Blunting” subscale (37.5%), and SANS “Anhedonia — Asociality” subscale (33.3%) in the first group.
When comparing the reduction in scores between the two groups, a greater therapy effectiveness was established in the group with DUI of less than 36 months. In particular, the depressive symptoms score on the HDRS scale (p=0.016; U=196.0) and prodromal symptoms score on the SOPS disorganization subscale (p=0.046; U=218.0) were down significantly. No other statistically valid differences were found (Figure 1).
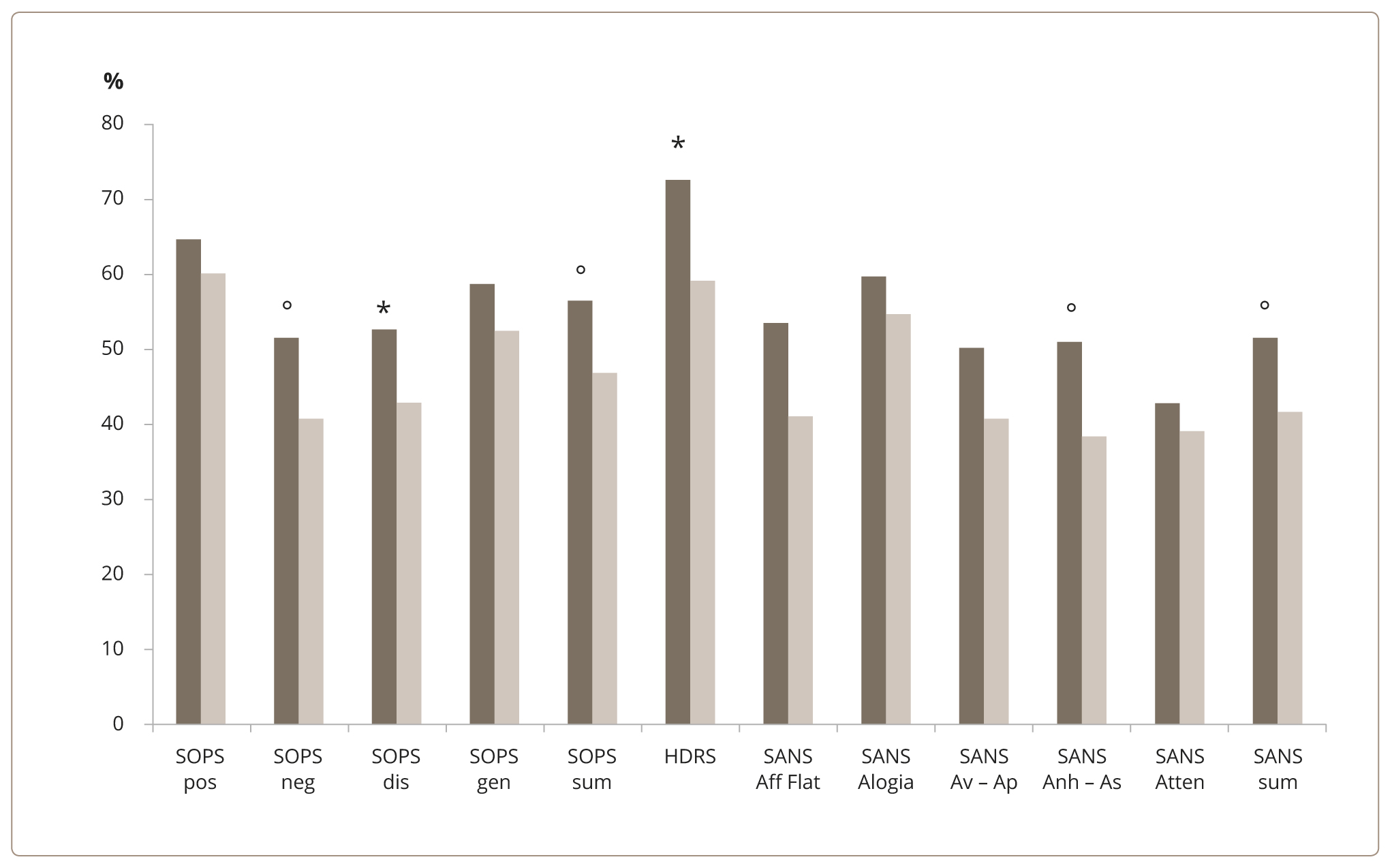 Figure 1. Degree of depressive and non-affective symptoms reduction (%) in the two groups of patients. Note: * — statistically significant (р <0.05); ° — trend towards statistical significance (0.05< р <0.1). Dark columns — the first group; light columns — the second group; pos — positive; neg — negative; dis — disorganization; gen — general; sum — summary; Aff Flat — Affective Flattening or Blunting; Av – Ap — Avolition – Apathy; Anh – As — Anhedonia – Asociality; Atten — Attention.
Figure 1. Degree of depressive and non-affective symptoms reduction (%) in the two groups of patients. Note: * — statistically significant (р <0.05); ° — trend towards statistical significance (0.05< р <0.1). Dark columns — the first group; light columns — the second group; pos — positive; neg — negative; dis — disorganization; gen — general; sum — summary; Aff Flat — Affective Flattening or Blunting; Av – Ap — Avolition – Apathy; Anh – As — Anhedonia – Asociality; Atten — Attention.
Across the results collected at the time of admission, positive correlations were found between DUI and the “Alogia” subscale of SANS (r=0.333, р <0.05), the degree of positive symptoms of SOPS (r=0.284, р <0.05), the symptoms of disorganization of SOPS (r=0.274, р <0.05), and the total HDRS score (r=0.313, р <0.05). Across the results collected before discharge, positive correlations were determined between DUI and the “Alogia” subscale of SANS (r=0.376, р <0.05).
The duration of active treatment was 36.9±18.5 and 47±31.9 days, for the first and second groups, respectively. No significant difference between the groups (p >0.05) depending on the duration of active treatment was found. Based on the UKU Side Effect Rating Scale, no significant side effects were identified (for all items in the scale, the values are 0).
Discussion
Main result
The study determined that the severity of depressive and non-affective symptoms at the time of admission does not depend on the duration of the DUI. However, the DUI has an impact on the reduction of depressive, negative symptoms, and symptoms of disorganization in young patients with a first depression episode. A high level of comorbidity has been found, confirming that a variety of non-psychotic and psychotic disorders in youth manifest themselves in depression at a prodromal stage, whereas no nonspecific affective symptoms are observed. High comorbidity with other mental disorders requires subsequent verification and underlines the difficulty of diagnosing young patients at their first depressive episode. In addition, it has been established that DUI has an impact on the taming of depressive symptoms upon discharge.
Strengths and limitations of the study
Our study exhibits the following strengths. Firstly, a holistic approach to the assessment of youth depression was adopted, allowing us to assess the dynamics of symptoms during treatment. Furthermore, it is clear that not only depressive symptoms, but also negative symptoms and symptoms of disorganization have clinical value in the context of DUI assessment.
The study has several limitations. Firstly, only male patients were included. Secondly, the sample size was relatively small, which may have potentially reduced the statistical potency of the study. Thirdly, follow-up of patients after discharge from the hospital was not performed, limiting the possibilities to analyze the dynamics of remission and its completeness. Finally, inclusion of patients with various types of depressions within nonpsychotic mental disorders may also have influenced the results.
Comparison with the existing literature
The obtained data do not support the results of other studies in which a relationship between the DUI and severity of the depressive episode was established [23]. This may be related to the pathoplastic effect of youth and high stress reactivity of this age, which determine the severity of clinical symptoms [24]. However, significant differences were found between the selected groups during the assessment at the stage of discharge from the hospital. Patients with a shorter DUI (less than 36 months) had a broader reduction of depressive symptoms in a relatively short period of therapy. This is consistent with early studies showing that delayed beginning of therapy reduces the likelihood of achieving full remission [4]. In addition to the higher level of depressive symptoms, patients with a longer DUI (more than 36 months) demonstrated a lower degree of reduction and a higher severity of negative symptoms, both on the SOPS scale and the SANS scale, symptoms of disorganization on the corresponding subscale SOPS, and a higher total score on the SOPS scale at discharge. To our knowledge, this is the first study to measure links between DUI and prodromal symptoms.
The pathogenesis of negative symptoms has now been found to be related to the functional reduction in dopamine levels in the frontal lobe and mesolimbic structures [25], and in the dorsal, rather than limbic, striatum [26]. The areas of the brain involved in cognitive dysfunction include the hippocampus, dorsolateral prefrontal cortex, and dorsal parietal cortex [27]. In depression, the main biological processes can be characterized by reduced neurotrophic support, metabolic dysfunction, impaired immune response with increased inflammation, oxidative stress, and mitochondrial dysfunction [28]. Thus, according to existing research, prolonged DUI has a long-term neurotoxic effect on the brain, which is manifested in increased ventricle volumes, atrophy of the cortex, white matter lesions in the frontal cortex and basal ganglia, as well as a decrease in the volume of the hippocampus [29]. Clinically, this can manifest itself as persistent negative symptoms and symptoms of disorganization, which can be difficult to treat. Interestingly, in the present study we found no cross-group differences in the reduction in positive symptoms. This is consistent with the dopaminergic hypothesis linking the appearance of positive symptoms to changes in the neurotransmission of dopamine in the mesolimbic system, with an increase in presynaptic regions [30], which is easy to treat and does not have a lasting effect on brain functioning.
Implications for future research and practice
The lack of a reduction in depressive, negative, and disorganized symptoms in the first group of patients with a longer DUI points to the need for more elaborate studies of youth depression, with a clarification of its pathogenesis. In addition, more attention should be given to depressive symptoms as predictors of adverse outcomes in mental disorders.
Conclusion
Our study showed that the DUI has an impact on the reduction of depressive, negative symptoms, and symptoms of disorganization in youth patients at the first depressive episode. A high level of comorbidity has been uncovered, confirming that a variety of nonpsychotic and psychotic disorders in youth manifest themselves in depression at a prodromal stage, causing difficulties in establishing diagnoses and requiring subsequent verification. Future research might need to focus on exploring depressive symptoms as predictors of mental disorders in youth patients.