INTRODUCTION
Schizophrenia is a chronic mental disorder that affects approximately 1% of the global population [1]. In 1911, Bleuler identified affective disorders as one of the core symptoms in his “4A” framework (associations, affect, ambivalence, autism) for schizophrenia [2], a concept that continues to be recognized in modern diagnostic classifications. For instance, the International Classification of Diseases, 10th revision (ICD-10), includes both depressive and psychotic symptoms under the following categories [3]:
- F31.5 — Bipolar disorder, current episode depressed, severe, with psychotic features;
- F25.1 — Schizoaffective disorder, depressive type;
- F20.4 — Post-schizophrenic depression;
- F32.3 — Severe depressive episode with psychotic symptoms;
- F33.3 — Major depressive disorder, recurrent, severe without psychotic features.
When considering the overlap between affective and psychotic symptoms, most of the research focuses on schizoaffective disorder, which encompasses depressive episodes in the context of schizophrenia [4, 5]. However, depressive symptoms do not always meet the full diagnostic criteria for a depressive episode, which is why this review explores depressive manifestations of schizophrenia.
The transition from Liddle's two- (positive and negative symptoms) and three-factor model (positive symptoms, negative symptoms, and disorganization) of schizophrenia [6] to the five-factor model underscores the distinct role and significance of depressive symptoms in the disease’s structure. Reflecting on evolving diagnostic paradigms, Mosolov et al. [7] highlight the independence and value of the dimensional model in psychiatry. The 5th edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) [1] outlines 8 clinical dimensions for schizophrenia spectrum and other psychotic disorders:
- hallucinations;
- delusional beliefs;
- disorganized speech;
- abnormal psychomotor behavior;
- negative symptoms;
- impaired cognition;
- depression;
- mania.
ICD-11 [8] identifies domains of schizophrenia and other primary psychotic disorders similar to dimensions (used as additional codes): positive, negative, depressive, manic, psychomotor, cognitive. Thus, the importance of identifying depressive symptoms is recognized in both classifications [9].
The relationship between schizophrenia and depressive symptoms is bidirectional: the presence of depressive symptoms worsens the course of schizophrenia, while a more severe progression of schizophrenia increases the likelihood of developing depressive symptoms [10]. The reported prevalence of depressive symptoms among individuals with schizophrenia varies widely, from 6% to 75% [11], depending on factors such as study design, disease stage, and diagnostic methods [12]. On average, depressive states occur in about 25% of patients with schizophrenia, a rate higher than that in the general population [13]. Additionally, depressive symptoms can manifest themselves at any stage of schizophrenia [14] and tend to be more severe in males [15]. These are often the most common and persistent symptoms of schizophrenia [16], contributing to reduced social functioning [10, 17], strained family relationships, and poorer treatment adherence [18]. Vauth et al. found that two-thirds of patients with schizophrenia are unable to fulfill basic social roles, and that only one-third are employed, often in positions requiring less qualifications than those they held before the onset of the illness [19]. As a result, depressive symptoms in schizophrenia contribute to dysfunction in the conduct of daily tasks [20]. Furthermore, the presence of depressive symptoms increases the risk of suicide in individuals with schizophrenia [21] and suicide is a leading factor in the reduced life expectancy of these patients [22], particularly in the early stages of the disease [23].
While the diagnosis of schizophrenia remained based on the presence of specific symptoms according to ICD-10 criteria [3], it also is a highly heterogeneous disorder. Although biomarkers are widely used in other areas of medicine, they remain absent for psychiatric conditions like schizophrenia [24]. Understanding the pathophysiology of depressive symptoms in schizophrenia could improve diagnosis and infrom more effective treatment choices [25].
The aim of this review is to analyze publications focused on identifying biological markers of depressive symptoms in schizophrenia.
METHODS
Eligibility criteria
Inclusion criteria:
- study participants had a confirmed diagnosis of “Schizophrenia” with depressive manifestations;
- studies aimed at identifying biological markers of depressive symptoms in schizophrenia;
- publication in English and/or in Russian;
- the period of publication is from January 01, 2018, to December 31, 2023.
Exclusion criteria:
- study participants had disorders comorbid with schizophrenia, including a depressive and/or manic episode.
Information sources
The search for papers was conducted using the following keywords in both Russian and English (as well as their combinations): “depressive symptoms”, “schizophrenia”, “neurobiology”, “biological markers”, “blood plasma”, “gene”. Articles from January 01, 2018, to December 31, 2023, were selected. This time period was selected to encompass the most relevant studies based on their recency. The group of authors conducted the information search process in turn and collegially. Each of the authors took equal part in the development of inclusion and exclusion criteria, data selection, information processing, and the writing of the literature review.
Search strategy
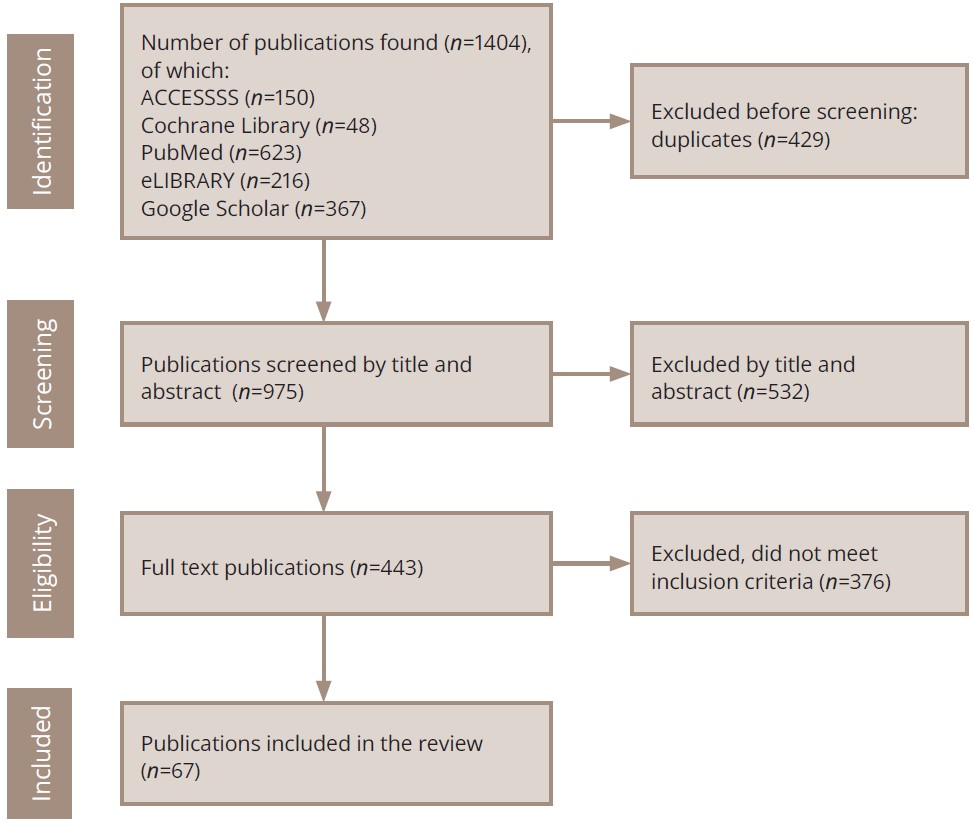
The search was conducted according to the principles of the clinical decision-making model “6S Hierarchy of EBM Resources”1 [26] using the service for the search for publications ACCESSSS from McMaster PLUS2 (Figure 1).
Figure 1. PRISMA flow diagram of the literature search and the selection process.
Source: Sultanova et al., 2024.
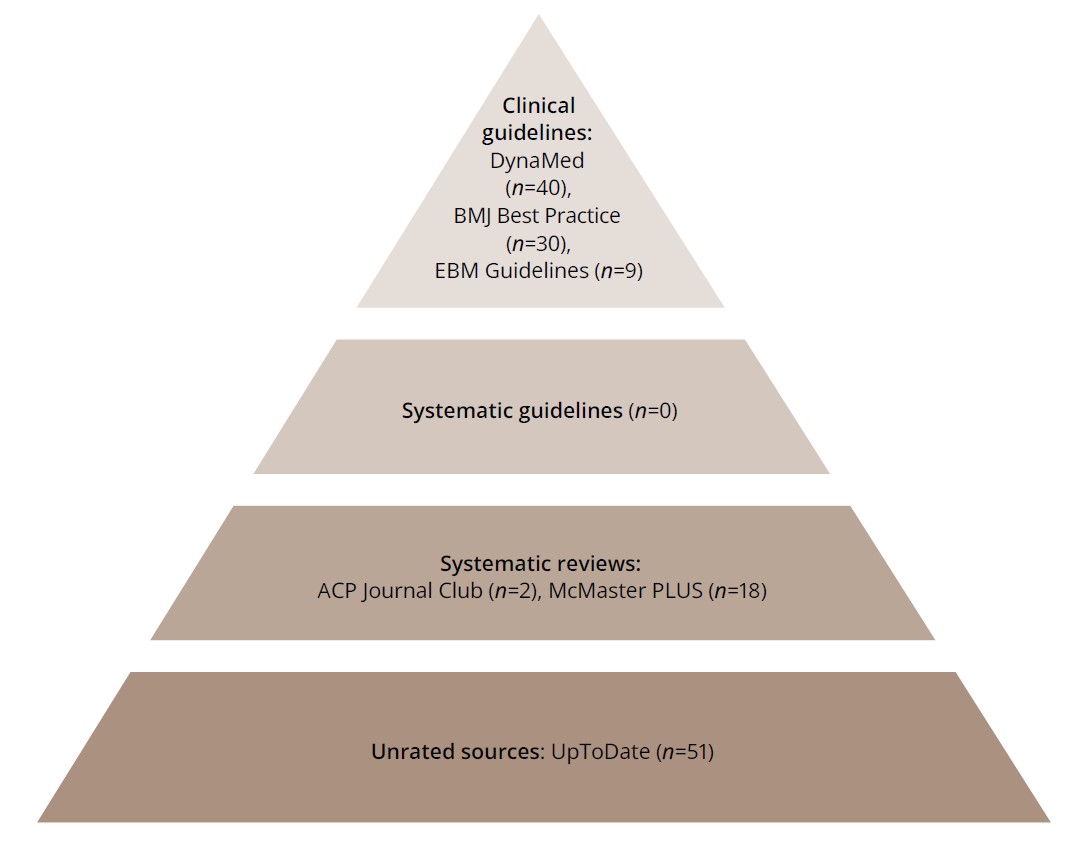
The structure of the “6S Hierarchy of EBM Resources” model is presented in Figure 2.
Figure 2. The 6S Hierarchy of EBM Resources model using McMaster PLUS’s ACCESSSS.
Note: DynaMed3 is a medical information Internet resource; Best Practice4 is an information Internet resource that physicians can use to make clinical decisions; EBM Guidelines5 are clinical guidelines based on the principles of evidence-based medicine; ACP Journal Club6 is a medical
publication that publishes current literature on internal medicine; Mcmaster PLUS7 is a service of the research unit of the medical information of McMaster University (Canada); UpToDate8 is a medical Internet resource providing medical information based on evidence-based information.
Source: Sultanova et al., 2024.
The search resulted in the extraction of clinical guidelines from DynaMed (n=40), BMJ Best Practice (n=30), and EBM Guidelines (n=9), from which all papers were excluded by abstract and title (n=79), as none of them met the selection criteria. No systematic guidelines were identified, and the next stage of the search included systematic review-level studies (ACP Journal Club, n=2; McMaster PLUS, n=18). None of the studies met the inclusion criteria (n=20). Further screening was performed from unrated sources (n=51), including UpToDate, of which 4 articles met the criteria and were included in the current review.
One publication from the Cochrane Library (n=48) was included in the review9. Studies (n=839) were also extracted from the PubMed (n=623)10 and eLIBRARY (n=216)11 portals, of which 24 were included in the final version of the article (duplicates were excluded beforehand). The remaining sources were selected in Google Scholar (n=367), and 38 sources were included in the final version of the paper. The primary challenge in the search was ensuring the careful exclusion of studies where patients with schizophrenia had a comorbid depressive disorder, as this review focuses solely on publications addressing depressive manifestations at the symptomatic level.
Analysis of the results
Two reviewers independently reviewed all the verified documents. As a result, 67 papers were included in the current review. The publications found and selected for analysis were studied in full (full-text versions of the manuscripts). The data were analyzed from the perspective of utilizing biological methods to study depressive manifestations in schizophrenia. As a result, it was decided to divide the presented results into three blocks:
- biochemical factors of depressive symptoms in schizophrenia;
- neuroimaging methods of depressive symptoms in schizophrenia; and
- genetic predictors in patients with schizophrenia with depressive manifestations.
Statistical methods were not used for data analysis.
RESULTS
Biochemical factors of depressive symptoms in schizophrenia
The data collection process began with an examination of biochemical markers in blood plasma, as it is a widely accessible and commonly used research method. Faugere et al. found that elevated C-reactive protein levels were associated with the presence of depressive symptoms in patients with schizophrenia [27]. Another study revealed a link between depressive symptoms in schizophrenia and elevated metabolic parameters, particularly triglycerides and low-density lipoproteins [28]. It has been found that, the peptide hormone leptin may influence the appearance of depressive symptoms in schizophrenia, with significantly higher levels observed in patients with depressive manifestations compared to healthy individuals. Moreover, leptin levels were shown to negatively correlate with the depression factor on the PANSS (Positive and Negative Syndrome Scale), though not on other subscales [29]. Other researchers identified a correlation between a higher dietary intake of glutamic acid and increased depressive symptoms in non-obese adults with schizophrenia, while no such correlation was observed in obese participants [30]. Further study demonstrated a connection between adiponectin, a hormone involved in fatty acid breakdown and glucose regulation, and depressive symptoms in schizophrenia, with lower baseline adiponectin levels being significantly associated with higher baseline depressive symptoms [31].
Research on biomarkers related to the body’s defense mechanisms revealed a correlation between depressive symptoms and the antioxidant enzymes manganese superoxide dismutase and total superoxide dismutase activity in the blood plasma of patients experiencing their first episode of untreated schizophrenia [32]. In a study by Bigseth et al., elevated levels of the soluble urokinase plasminogen activator receptor (suPAR) were linked to depressive symptoms in women with schizophrenia, suggesting abnormal immune activation within this subgroup [33].
Brain-derived neurotrophic factor (BDNF), a key modulator of neuroplasticity, is implicated in the pathogenesis of both schizophrenia and depression [34]. Although BDNF is not a specific synaptic molecule, research has shown that reduced serum BDNF levels in patients with schizophrenia are associated with more severe depressive symptoms [35], particularly in the early stages of the illness [36]. Han et al. study of patients with schizophrenia and depressive symptoms who were on monotherapy with olanzapine for 12 weeks demonstrated a significant increase in BDNF levels and a reduction in depressive symptoms following treatment [37]. Additionally, it was found that the concentration of another neurotrophic factor, neurotrophin-3 (NT-3), increased in the serum of patients with schizophrenia only in the presence of depressive symptoms, with no significant difference in NT-3 levels between schizophrenic patients and a control group in the absence of depression [38].
Neuroimaging methods of depressive manifestations in schizophrenia
Neuroimaging is a key research tool in schizophrenia studies. In a meta-analysis of 4474 patients with schizophrenia and 5098 controls, brain imaging of cortical thickness and surface area abnormalities revealed a decreased gray matter volume in regions crucial to both schizophrenia [39] and depression [40]. Specifically, a reduced gray matter volume in the prefrontal cortex, including the medial part of the right superior frontal, medial orbitofrontal, and superior and middle frontal gyri, was linked to depressive symptoms in schizophrenia patients. Notably, depressive symptoms in schizophrenia had a more significant impact on gray matter reduction than negative symptoms [41]. According to magnetic resonance morphometry, a comparison between schizophrenia patients with/without depressive symptoms with a healthy control group indicated pronounced gray matter abnormalities concentrated in the cingulate gyrus in schizophrenia patients with depressive symptoms [42]. The reduction in gray matter was most significant in first-episode schizophrenia patients with depressive symptoms, especially in the parietal, frontal, and temporal lobes, including Broca’s and Wernicke’s areas, and the prefrontal cortex [43]. In comparisons of patients with depressive episodes and psychotic symptoms and those with schizophrenia and depressive symptoms, the latter group showed reduced gray matter in the left tegmental area and left frontal lobe [44].
Although electroencephalography (EEG) is not a neuroimaging method, a study by Shor et al. using EEG demonstrated that patients with both schizophrenia and depression exhibit lower hierarchical interconnectivity and reduced information segregation compared to healthy controls [45].
In animal studies, in vivo two-photon calcium imaging and electrophysiological recordings, combined with behavioral phenotyping, were used to investigate schizophrenia models. To create a mouse model of schizophrenia, the animals received dizocilpine (an N-methyl-D-aspartate, NMDA antagonist); depressogenic factors were created three days after the injection by tilting the cage, wet bedding, forced swimming, physical restraint, and sleep deprivation. The study found that schizophrenia with depression is expressed by a variety of symptoms, including helplessness, anhedonia and reduced filtering of sensory information, having worse indicators for these symptoms than depression or schizophrenia alone. These behavioral deficits were associated with disrupted neuronal calcium activity in the frontal cortex and thalamic nuclei [46, 47].
Genetic predictors in schizophrenia patients with depressive symptoms
Research on candidate genes has identified a number of common genetic factors associated with depressive manifestations in schizophrenia. One such gene is the methylenetetrahydrofolate reductase (MTHFR) gene [48]. In our study, while we did not uncover an association with the MTHFR C677 polymorphism, we found that elevated homocysteine levels may serve as a risk factor for the development of depressive symptoms in patients with schizophrenia [49].
Low levels of BDNF and SIRT1 have also been implicated in the emergence of depressive symptoms in schizophrenia [50]. However, the BDNF gene contains multiple single nucleotide polymorphisms (SNPs) that exhibit strong linkage disequilibrium and interact in ways that may affect susceptibility to a mental illness. This complexity suggests the need for further detailed investigations of the gene [51]. The SIRT1 gene encodes a protein belonging to the sirtuin family, whose functions are not yet fully understood. However, emerging evidence suggests that this gene may play a role in the pathogenesis of depression [52]. Preliminary findings from one study indicate that the SIRT1 gene may increase the susceptibility of patients with schizophrenia to depressive symptoms [53].
Oxytocin, a regulator of social and emotional behavior, is a promising candidate for assessing susceptibility to schizophrenia. Research on schizophrenia patients suggests that oxytocin may play a role in the cognitive and social deficits development, possibly due to overactivation of the hypothalamic-pituitary-adrenal (HPA) axis, a known contributor to neurodegenerative changes in schizophrenia. Given its involvement in emotion and facial expression recognition, oxytocin has become a focal point for studying the pathophysiology of negative and depressive symptoms in schizophrenia, particularly reduced emotional expression. A study by Broniarczyk-Czarniak et al. demonstrated a correlation between the expression of the OXT gene, at both the mRNA and protein levels, and the severity of depressive symptoms in schizophrenia patients [54].
The impact of lipid metabolism disorders in schizophrenia patients has attracted particular attention from researchers. They have proposed a genetic link between depressive symptoms and lipid metabolism. Preliminary evidence indicates that the rs7754840 polymorphism of the CDKAL1 gene, which is crucial for lipid metabolism, may contribute to the development of depressive symptoms in drug-naive schizophrenia patients [55]. Additionally, a study by Li revealed that the apolipoprotein E gene (APOE E2) is associated with the emergence of depressive symptoms in schizophrenia patients [56].
DISCUSSION
Brief interpretation of results
In this literature review, we consolidate the disparate data on biological markers of depressive symptoms in schizophrenia. Upon reviewing blood parameters, it becomes evident that this method cannot currently be considered specific for diagnosing depressive manifestations in schizophrenia. Changes in the concentration of proteins, such as C-reactive protein, soluble urokinase plasminogen activator receptor (suPAR), enzymes (superoxide dismutase), metabolic markers (triglycerides and low-density lipoproteins), and hormonal profile markers (leptin and adiponectin), are also observed in other conditions.
Analyzing the literature data on blood parameters, we obtained information on the influence of neurotrophic factors. For instance, NT–3 levels were significantly elevated, while BDNF levels were decreased in patients with schizophrenia experiencing depressive symptoms. However, these findings cannot be currently used as a diagnostic feature as they lack specificity, as reduced BDNF levels are also associated with several other psychiatric disorders. Based on current evidence, it can be concluded that blood parameters cannot yet be used as specific markers for identifying depressive symptoms in schizophrenia, prompting an attempt to explore other research methods.
Clarifying the neural mechanisms by which depressive symptoms exacerbate the course of schizophrenia is essential for identifying new therapeutic targets and developing treatment strategies [57]. In a study of depression and psychosis, Lalousis et al. [58] suggested that a neurobiologically driven approach is more effective in predicting symptomatic and functional remission than traditional diagnostic categories in predicting the course of the disease. A reduction in the gray matter volume [41] has been observed in the parietal, frontal, and temporal lobes, particularly in Broca’s and Wernicke’s areas [43], as well as in the prefrontal cortex, including the medial part of the right superior frontal, medial orbitofrontal, and superior and middle frontal gyri [42, 44]. Several studies have confirmed gray matter reduction in cases of depressive symptoms in schizophrenia, though some findings require further confirmation through additional research [45–47].
The results of the analysis of candidate genes that contribute to the development of depressive symptoms in schizophrenia were also presented. The SIRT1 [50, 53], OXT [54], CDKAL1 [55], and APOE [56] genes may contribute to the development of depressive symptoms in a sample of patients with schizophrenia. However, findings on the MTHFR gene remain inconsistent and require further investigation [48, 49]. Although genetic studies have limitations, the search for genetic associations represents one of the most promising approaches for identifying biological markers, particularly in patients with depressive symptoms in schizophrenia. An equally important issue for clinicians is the differential diagnosis of depressive symptoms in schizophrenia, which can be complicated by adverse reactions to antipsychotic medication [59, 60], substance abuse [61], and psychosis-related manifestations that may induce depression [62, 63]. A key challenge in diagnosis is distinguishing between depressive and negative symptoms [64]. Some researchers posit that negative symptoms are not exclusive to schizophrenia but may also occur in depressive disorders [65]. Negative symptoms can be secondary to depression or overlap with depressive features [66]. Other researchers emphasize certain shared characteristics, such as anergia, poverty of speech, and anhedonia, while recommending a symptom-based approach for differentiation: depression is associated with hopelessness, pessimistic and suicidal thoughts, while negative symptoms are characterized by blunted affect, poverty of speech, objective depression, social withdrawal, and reduced attention span [57]. Nevertheless, network analysis has shown that depressive symptoms in schizophrenia are weakly correlated with negative symptoms, making them relatively distinguishable [67]. The complexities inherent in differentiating depressive manifestations in schizophrenia result in many patients receiving inadequate treatment, underscoring the need for further research into biological markers [29].
Limitations
One limitation of this review is the six-year publication window for the analyzed studies. The studies included were notably heterogeneous, with varying methods used to diagnose depressive symptoms in schizophrenia. The sample comprised both treatment-experienced and naive patients, which adds to variability. While the data on biological markers show promise for research, these markers must undergo standardization, sensitivity and specificity testing, and validation before they can be introduced into clinical practice. The practical relevance of these findings lies in the potential for neurobiological methods to improve the diagnosis of depressive symptoms in schizophrenia, enhancing research practices and facilitating psychiatrists’ clinical work. This is particularly relevant given the challenges in differentiating depressive symptoms from negative symptoms, which remain significant. Early detection of depressive symptoms is also crucial for preventing severe complications, such as completed suicide attempts. Overall, the review underscores the considerable potential of neurobiology in diagnosing depressive manifestations in schizophrenia. The adoption of these methods could lead to significant improvements in diagnosis, treatment, and, ultimately, the quality of life for these patients.
CONCLUSION
In recent years, numerous studies have explored the neurobiological aspects of depressive symptoms in schizophrenia. However, it remains premature to implement these diagnostic methods in routine clinical practice. Continued research into biological markers will provide greater clarity about the development of depressive symptoms in schizophrenia and will influence the choice of priority treatment methods.
1 6S Hierarchy of EBM Resources is a hierarchy of resources (information sources) based on evidence-based medicine and provides a model for guiding clinical decision-making.
2 Available from: https://www.accessss.org/
3 Cochrane [Internet]. London: Cochrane [cited 2024 Aug 26]. Available from: https://www.cochrane.org
4 PubMed [Internet]. Bethesda: National Center for Biotechnology Information, U.S. National Library of Medicine [cited 2024 Aug 26]. Available from: https://pubmed.ncbi.nlm.nih.gov
5 eLIBRARY.RU [Internet]. Moscow: Scientific Electronic Library [cited 2024 Aug 26]. Available from: https://elibrary.ru
6 DynaMed [Internet]. Ipswich (MA): EBSCO Information Services. 1995 [cited 2024 Aug 26]. Available from: https://www.dynamed.com
7 BMJ Best Practice — a resource that physicians can use to make clinical decisions [Internet]. London: BMJ Publishing Group Limited [cited 2024 Aug 26]. Available from: https://bestpractice.bmj.com/info
8 EBM — Evidence-Based Medicine Guidelines [Internet]. Helsinki: Duodecim Medical Publications Ltd [cited 2024 Aug 26]. Available from: https://www.ebm-guidelines.com/apps/dtk/ebmg
9 ACP Journal Club — a medical publication that publishes current literature on internal medicine [Internet]. Philadelphia: American College of Physicians [cited 2024 Aug 26]. Available from: https://www.acponline.org/clinical-information/journals-publications/acp-journal-club
10 McMaster PLUS — a service of the research unit of the medical information of McMaster University (Canada) [Internet]. Hamilton: McMaster University [cited 2024 Aug 26]. Available from: https://plus.mcmaster.ca/McMasterPLUSDB
11 UpToDate provide medical information based on evidence-based information [Internet]. Waltham: UpToDate, Inc. [cited 2024 Aug 26]. Available from: https://www.uptodate.com/login