INTRODUCTION
The need for a scientific understanding of how the brain ages is rooted in the current demands in society for an extended active life. Researchers are convinced that not only the pathological, but also compensatory mechanisms of aging should be taken into account in order to maintain intellectual longevity [1]. According to Stern et al., the cognitive reserve (CR) is one of the protective mechanisms against clinically significant cognitive decline, even in the presence of neurodegeneration [2]. Not only Stern, but other authors as well note that the CR increases brain efficiency and intellectual productivity [3]. Individuals with a high CR are resistant to clinical manifestations of Alzheimer’s disease (AD) and other neurodegenerative diseases [4, 5]. Advanced brain studies have shown that some elderly people retain their cognitive capacity through life despite suffering significant cerebral atrophy and degeneration [6]. Such significant differences between degrees of brain damage and severity of clinical signs (or absence thereof) stand at the basis of the CR concept [7].
It is now recognized that pathophysiological changes begin many years prior to the appearance of clinical manifestations of the disease and that the spectrum of AD spans from clinically asymptomatic to severely impaired individuals [8]. Advances in biomarker research have furthered our understanding of AD as a structurally complex process moving along an unbroken continuum [9, 10]. The pathophysiological basis of the AD continuum is the multifactorial etiology and pathogenesis of the disease [11]. Biomarkers such as gene mutations, amyloid and tau pathology [12, 13], neuroinflammation, mitochondrial dysfunction, and other pathological processes become involved in the multifactorial pathogenesis of AD decades before the onset of the first clinical symptoms of the disease and are responsible for the stepwise, gradual disease progression [14, 15]. Some of these factors are currently considered AD biomarkers, appearing decades before the onset of clinical symptoms [16, 17].
Transition from the preclinical (latent) stage to symptomatic AD depends on the interaction between pathological and protective factors. The CR may prevent the transition of AD to the clinical stage in some carriers of AD biomarkers [18]. In other words, individuals differ in their ability to cope with changes associated with aging, disease, or brain injury. However, AD patients with similar disease manifestations and a comparable degree of cognitive decline may be experiencing different changes in brain morphology. The question then arises: “What is the degree of brain resistance to pathogenic stimuli?” The significance of brain repair mechanisms and the role of the brain and CR at the latent and clinically apparent stages of the disease also remain unclear.
The aim of our work was to review scientific publications that have investigated the mechanisms and functions of the brain and cognitive reserve (BCR) in AD patients.
METHODS
Eligibility criteria
Inclusion criteria:
- full-text publications (meta-analyses, original studies, descriptive reviews) selected using the keywords “cognitive reserve”, “Alzheimer’s disease”, “brain reserve”;
- publications selected for review had to contain a description, analysis, or results of studies that enrolled patients with AD diagnosed according to the criteria of the International Classification of Diseases, 10th revision (ICD-10), or the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5).
Exclusion criteria:
- participants included in the selected studies were not verified as AD patients according to the ICD-10 and DSM-5 criteria.
Information sources
Using the combination of the above-mentioned keywords, which provides a high-quality description of the content and increases the efficacy of the publications search, we carried out a descriptive review of 83 scientific publications. The aim of this review was to investigate the mechanisms and functions of the BCR in patients with AD. Publications were selected using the inclusion criteria (see above). The study materials were publications included in PubMed, the biomedical literature search engine, and eLIBRARY, the electronic library.
The scope of the search was not limited, since one of the objectives of this work was to define the terminological boundaries of the concepts of “cognitive reserve” and “brain reserve”. Therefore, the list of references includes works published 10 or more years ago.
A review of 12 meta-analyses published from 2012 to 2024 and identified in the PubMed database using the above-stated keywords is presented in the Results and Discussion sections of this work.
Search strategy
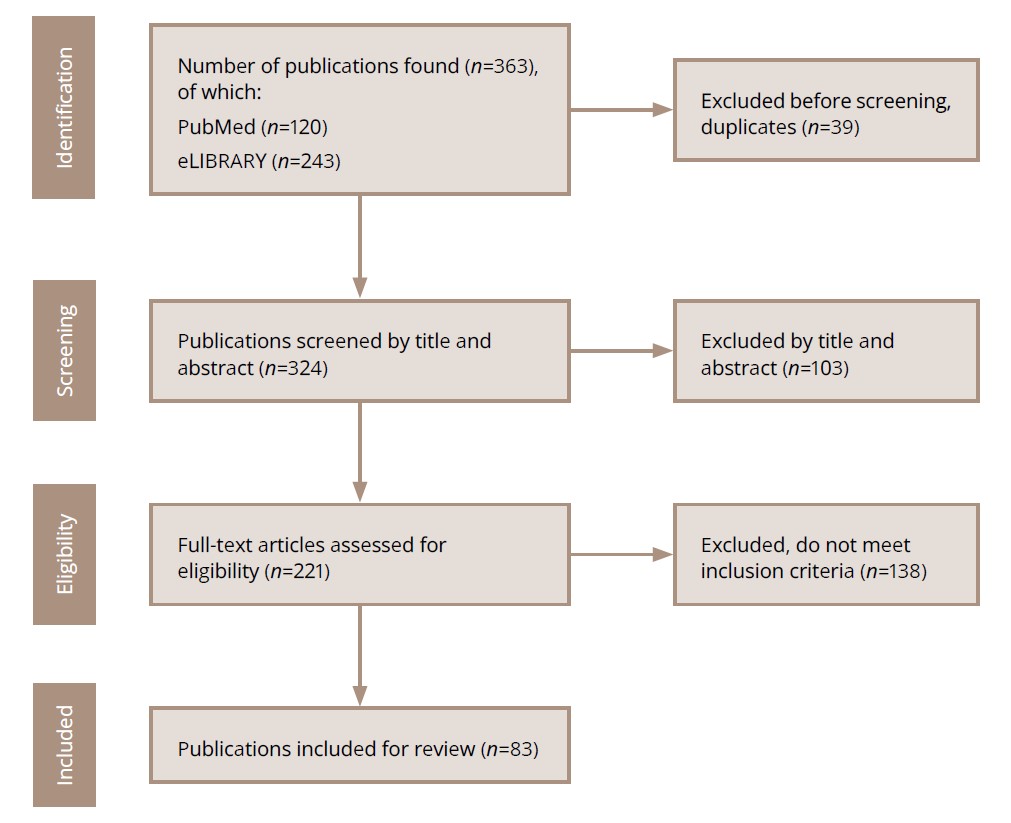
Publications were searched step by step. The search sequence is shown in Figure 1.
Figure 1. Steps in the search for publications to be analyzed.
Source: Sidenkova et al., 2024.
Selection process
Each publication was selected using a manual search. Several of the authors involved in this work performed the search and selection of publications (see “Authors’ contributions”). Some publications selected at the screening stage were subsequently excluded from further analysis, because they did not meet the inclusion criteria.
Analysis of the results
Each publication was analyzed. A synthesis of the information obtained from the selected scientific sources was performed. The results of the synthesis are presented in structured text, tables, and figures.
RESULTS
Concept of cognitive reserve
Some researchers working on neurodegenerative disorders seek to determine why some individuals retain their normal cognitive functions despite experiencing significant cerebral degeneration and to identify the mechanisms that trigger CR involvement. To answer this question, it appears necessary to clearly outline the terminological boundaries of the concept of CR (Table 1).
Table 1. Formation of the cognitive reserve concept
|
Authors |
Year of publication |
Definition of cognitive reserve (CR) |
|
Stern [19] |
2002 |
CR is a combined factor that is influenced by the accumulated life experience. It cannot be measured directly due to its multifactorial and dynamic nature. |
|
Soldan et al. [20] |
2017 |
CR is a theoretical, abstract concept suggesting that greater engagement in cognitively stimulating activities throughout life modifies the brain, thus reducing the negative impact of brain disorders on cognitive function. |
|
Stern et al. [21] |
2019 |
CR is an individual’s ability to optimize cognitive function through differential recruitment of brain structures or neural networks in brain activity. |
|
Soldan et al. [22] |
2020 |
CR is an ability of the brain to mitigate the sequelae of brain disorders or cognitive function impairment. |
|
Stern et al. [23] |
2022 |
The definition of CR includes two independent components: 1) assessment of brain damage affecting cognitive functions; 2) assessment of cognition; 3) measurement of the variable that affects steps (1) and (2). |
In our opinion, an exhaustive definition of CR has not yet been formulated, though the view of CR as a set of processes resisting neurodegeneration from its earliest preclinical stages allows us to answer the question of why some individuals successfully cope with progressive brain disorders, while others cannot tolerate the same level of brain damage.
To study and evaluate the brain repair mechanisms in neurodegeneration, it is necessary to know the features of its pathogenesis. An executive summary of current models of AD development is presented in Table 2.
Table 2. Dynamic models of Alzheimer’s disease
|
Authors |
Year of publication |
Description of an AD model |
|
Hypothetical model of dynamic biomarkers of the AD pathological cascade |
||
|
Jack et al. [24] |
2010 |
The sequential change of preclinical, prodromal, and dementia stages of Alzheimer’s disease is caused by the interaction of 2 types of biomarkers: - biomarkers associated with the presence of disease; - biomarkers associated with the stage (i.e. progression) of disease. |
|
Biomarker model of preclinical AD |
||
|
Sperling et al. [25] |
2011 |
This model reflects the cumulative nonlinear dynamics of several biomarker types: - reductions in Aβ42 in the cerebrospinal fluid and increased amyloid tracer retention on PET imaging are biomarkers of brain Aβ amyloidosis; - elevated CSF tau protein is a biomarker of neuronal injury; - decreased fluorodeoxyglucose 18 uptake on PET with a temporoparietal pattern of hypometabolism is a biomarker of synaptic dysfunction; - atrophy of medial temporal lobes, paralimbic and temporoparietal cortices on structural MRI is a biomarker of neurodegeneration. |
|
AD model of the National Institute on Aging and the Alzheimer’s Association Workgroup (NIA-АA), 2018 |
||
|
Jack et al. [26] |
2018 |
AD diagnosis should be based on combined (clinical and biomarker) diagnostic criteria. According to these criteria, the early preclinical (presymptomatic) AD stage is diagnosed if Aβ biomarker is positive in vivo. Symptomatic AD is confirmed by in vivo detection of Aβ and pathological tau, as well as neurocognitive impairment. |
|
Multi-marker model of AD (A/T/N) |
||
|
Lodder et al. [27] |
2021 |
This model takes into account the profiles of several biomarkers detected in evaluated patient: - “A” refers to amyloid pathology and is determined by the presence of Aβ42 or Aβ42/Aβ40 in the CSF or Aβ in the brain structures on PET imaging; - “T” refers to tau pathology and is determined by the presence of phospho-tau in the CSF or detection of abnormal tau filaments (intracellular thread-like tau structures) in the brain parenchyma on PET imaging); - “N” refers to neurodegeneration and is confirmed by the presence of tau in the CSF and in the brain parenchyma on MRI or 18F-fluorodeoxyglucose PET. |
Note: AD — Alzheimer’s disease; PET — positron emission tomography; MRI — magnetic resonance imaging; CSF — сerebrospinal fluid.
The authors of a meta-analysis of 17 functional MRI (fMRI) cohort studies reported a high probability of dementia in individuals with localized, increased activation of the left anterior cingulate cortex during cognitive tasks, compared with older adults who activate a broad network of brain regions during intellectual workload, including the medial and lateral frontal areas and the precuneus. That is, AD affects the frontoparietal network responsible for the cognitive control associated with general tasks. Detection of certain biomarkers, e.g. changes in specific fMRI activity and tauopathies, increases the probability of dementia by a factor of 2 [28]. The probability of dementia increases in neurodegeneration carriers. This finding has been confirmed in different study cohorts; for example, the probability of dementia stood at 54% in a cohort with brain damage confirmed by neuroimaging, compared with 26% in the control group [29]. However, clinical and neuropsychological assessments do not always identify dementia in patients with signs of neurodegeneration. Indeed, studies have shown that a significant proportion of community-dwelling older adults with advanced neurodegeneration do not develop dementia. This apparent disconnect is explained by the combined influence of several factors: genetic polymorphism, other brain disorders, slow disease progression, lifestyle factors, CBR volume, and premature mortality from concomitant diseases [30]: that is, the multimarker dementia model based on the risks of AD in a probabilistic aspect.
Concept of brain and cognitive reserves
Some researchers suggest distinguishing the brain (passive) reserve and the cognitive (active) reserve [21]. However, not all researchers agree with this approach and prefer the general concept of a single reserve, using the term CR [31]. We will nevertheless describe the mechanisms underlying the brain and cognitive reserves, according to different authors (Table 3).
Table 3. Mechanisms of brain reserve
|
Author |
Year of publication |
Concept |
|
Barnes, McNaughton [32] Norris et al. [33] |
1980 1996 |
Studies on animal models have shown that CR depends not on the number of neurons, but on their plasticity and the quality of connections between them. |
|
Katzman et al. [7] |
1988 |
The concept of CR includes such parameters as brain size, the proportion of healthy and abnormal neurons, and the structural integrity of neurons and synapses. This model defines brain reserve as an organ (physical) quality of the brain: some people have larger brains, with more neurons and synapses, which, according to researchers, maintains the brain’s resilience to damage, preventing cognitive dysfunction. |
|
Kunkle et al.[34] |
2019 |
CR becomes evident in the settings of neuropil loss, manifested by axon shortening and dendrite thinning. This results in loss of pathways transmitting signals between neuronal bodies, while a more powerful signal than normal is transmitted through the remaining synapses. This results in neuronal hyperexcitability. |
|
Soldan et al. [35] |
2020 |
CR is a morphological concept reflecting the structural properties of the brain that ensure its ability to maintain cognitive functions despite the significant loss of their material substrate. |
Note: CR — сognitive reserve.
A multicenter observational study on predementia Alzheimer’s disease performed at the German Center for Neurodegenerative Diseases demonstrated that large volumes of hippocampal subfields, particularly CA1, may serve as a brain backup system that ensures normal cognition and absence of subjective cognitive decline in patients with amyloid pathology [36]. The study also showed that this effect does not depend on the education level, or psychological or social characteristics of the participants [36]. On the contrary, other authors show that CR mechanisms are closely associated with the tau pathology and tau deposition in the medial and inferior temporal lobes [37].
Some authors believe that CR is a property of the brain that allows for cognitive performance that is better than expected given the degree of brain damage associated with neurodegeneration, traumatic brain injury, or other diseases [38]. Meta-analyses of cohort studies on CR localization in healthy aging, AD, and mild cognitive disorders, such as mild cognitive impairment (MCI), using functional MRI and positron emission tomography (PET) of the brain, demonstrated that in healthy and pathological aging CR is mediated by different brain regions [39]. In healthy older adults, the same cognitive tasks are associated with the activation of a broad network of brain regions, including the medial and lateral frontal regions (anterior cingulate cortex, dorsolateral prefrontal cortex, precuneus). In patients with AD and amnestic MCI, successful completion of the same cognitive task is associated with isolated activation of the anterior cingulate cortex [40]. A positive correlation between the brain volume and CR has also been demonstrated [41]. In a systematic review, Harrison et al. defined CR as the ability to use more efficient and flexible cognitive strategies, and engaging alternative networks, which can be enhanced through continuous cognitive training [42].
Meta-analyses show that CR is influenced by numerous environmental factors and individual psychological features, such as gerotranscendence, psychological well-being, the coping strategies used, and lifelong self-regulation strategies [43].
The following combination of morphofunctional and psychosocial factors makes cognitive (active) reserve possible:
- The morphofunctional characteristics of brain cells include a relatively increased size of the neuron body, a large number of axons, synapses, intensive DNA and RNA synthesis, and active functioning of presynaptic receptors [28].
- The psychological and social factors developed during lifetime are intelligence indicators, the level of education, professional affiliation, the volume of leisure activities, as well as the cognitive, communicative, social, and motivational activity of an individual [44–46].
According to current knowledge, the brain and cognitive reserves are not mutually exclusive. The dynamic capacity and structural characteristics of the neural network determine the quality of brain functioning in the settings of age-related changes and cerebral disorders. A systematic review and meta-analysis by Nelson et al. convincingly demonstrate that a higher CR is associated with a lower relative risk of MCI or dementia progression, reducing the risk of symptomatic AD almost twofold (47%) [47]. These results suggest that CR delays the onset of MCI and dementia in AD, and, therefore, serves as a potential target for preventive interventions.
The models of brain and cognitive reserves reflect the substrate and level of brain functioning, and represent a BCR. BCR is dynamic and depends on environmental factors and an individual’s life experiences throughout their lifespan.
Functions of brain and cognitive reserve
The brain and cognitive reserve reduce the negative impact of degeneration on the brain function in several ways described below.
BCR reduces MCI or dementia risk through mechanisms independent of the degree of neurodegeneration [48].
BCR interacts with markers of brain pathology or health, influencing future cognitive decline or the risk of disease progression. The protective effects of BCR decrease as the number of damaged neurons increases [49].
The protective effect of BCR increases with later AD onset and a lower rate of damaged substrate accumulation [50].
In individuals with a high reserve, neurodegeneration is less likely to affect the brain structure and function compared to those with a low reserve [51].
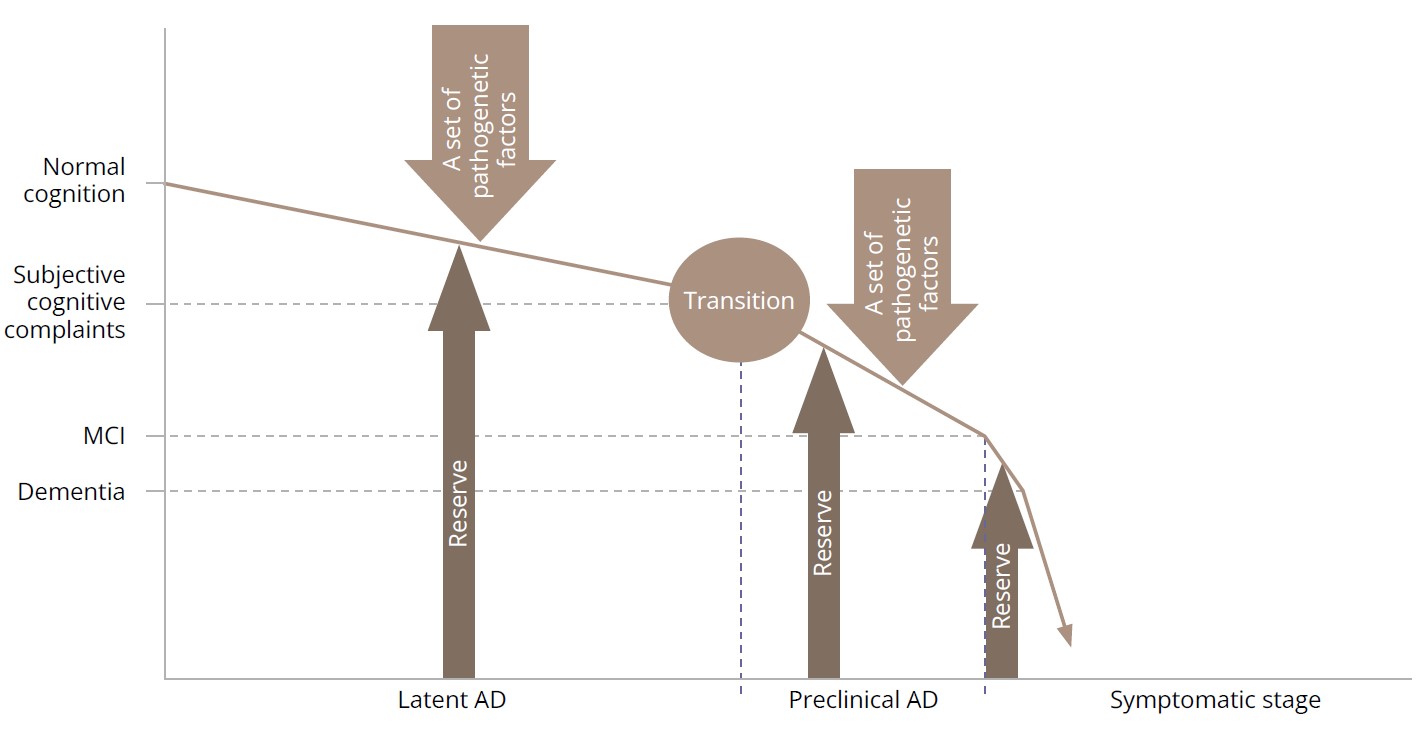
Data from the studies included in our meta-analyses suggest that the roles of the reserve are different in aging and neurodegeneration (Figure 2).
Figure 2. Brain and cognitive reserve in Alzheimer’s disease.
Note: MCI — mild cognitive impairment; AD — Alzheimer’s disease. Source: Sidenkova et al., 2024.
BCR mechanisms prevent progressive depletion of the regulatory function of the cortex, support motivational and behavioral activity, ensure functional hemispheric asymmetry, and have a neuroprotective impact during normal aging [52, 53].
BCR mechanisms support the activity of the frontal lobes and the hippocampus, and they compensate for dysfunction of these regions, allowing for the preservation of cognitive regulatory functions at preclinical AD stages, presumably thanks to the relatively larger area of the Brodmann hippocampal subfields (CA1/CA2/CA3 and subiculum), as was demonstrated in the functional MRI study in AD patients [54].
BCR delays clinical manifestations of AD until the reserve is depleted. In individuals with a large BCR volume, AD onset is characterized by pronounced symptoms, a high rate of progression, and a high incidence of affective and behavioral disorders. This is due to the fact that neurodegeneration is restrained by the reserve, but when it is depleted, this process manifests itself as marked synaptic and cholinergic neuronal dysfunction [55]. At this stage of the disease, BCR acts as a compensation mechanism aimed at reorganizing brain resources [39].
Cognitive continuum
Another meta-analysis demonstrated that in elderly patients with AD and MCI, previously active, coordinated and extensive neural networks stop functioning, and that task performance induces the activation of energy-consuming associative connections in the frontotemporal regions, compensating for the lost capabilities of the previously extensive healthy networks [56]. In their meta-analysis, de Las Fuentes et al. explained the faster cognitive decline in patients with high BCR by the older age of the participants, and, consequently, significant accumulation of amyloid and the tau protein at symptoms onset, as well as by a high incidence of age-related concomitant diseases; e.g., cerebrovascular disorders [57]. BCR appears to delay the onset of clinical symptoms associated with underlying AD.
Our understanding of the BCR concept is supported by the analysis of certain typical clinical situations: e.g. extremely fast progression of dementia in highly educated individuals engaged in active intellectual work. Some experts believe that the educational level is a type of protection against severe cognitive impairment [58]. According to this view, the above-mentioned example of the extremely fast AD progression into severe dementia represents an inexplicable exception. In his book “Problems of Causality in Medicine”, Davydovsky stated that any disease results from an interaction between a combination of pathogenic processes and protective and adaptive mechanisms aimed at restoring the impaired body self-regulation [59]. The actual manifestation of the disease is due to the failure of protective elements, when the disease breaks out. The authors of the Rotterdam study of patients with cerebrovascular disorders also came to an interesting conclusion: The lower risk of dementia in highly educated study participants could be explained by a higher BCR [60]. From this point of view, symptoms of dementia develop when the BCR depletes below a certain threshold. A smaller initial BCR would mean that less change would be required to reach the dementia threshold at which impairment would be evident, whereas a larger BCR would presumably provide greater protection against dementia. According to this theory, BCR may reflect either innate differences in cognitive abilities determined by the characteristics of prenatal synaptogenesis or postnatal maturation of brain structures (myelination, synaptic sprouting, development of hierarchical connections within the brain, etc.), which underlies the quality of cognitive processes. In any case, the level of education is an indicator of a higher BCR [61].
DISCUSSION
Investigation of the mechanisms of AD pathogenesis allowed modern researchers to come to the conclusion that disease manifestations are the result of an interplay of two countervailing processes: traditionally widely studied neurodegeneration and the brain repair mechanisms, represented by the BCR. The aim of this review was to summarize the results of meta-analyses and original studies on the mechanisms and functions of the BCR in patients with AD. The BCR concept helps us understand why not all individuals with preclinical AD transition to the symptomatic disease, despite the development of a pathological process confirmed by biomarkers. According to some authors, this transition occurs when the protective brain repair mechanisms fail and are no longer able to maintain the body’s homeostasis in the settings of progressive brain damage caused by neurodegeneration [62, 63–66]. As the number of damaged neurons increases (as evidenced by amyloidosis and tauopathy), the protective role of the ВCR that passively maintains the brain resilience to damage recedes [67]. Acceleration of disease progression and worsening of cognitive impairment, confirmed by morphological biomarkers, change the protective function of BCR into a compensatory one, which manifests itself by irrational, energy-consuming, widespread involvement of intact brain structures during cognitive tasks [68, 69]. BCR involvement in the general AD scenario implies that it is a factor, or a group of factors, capable of altering the expected course of a neurodegenerative disease [70, 71].
We believe that the BCR is formed long before brain aging. Robitaille et al. showed that the level of intelligence before the disease onset and the quality and type of leisure activity are inversely correlated with the resting regional metabolic activity of the brain and cerebral blood flow in different cortical and subcortical regions [72]. This suggests that the differences in dementia onset may have to do with the individual features of environmental and social factors not only in adulthood and old age, but also in childhood and adolescence. Liberati et al. believe that CR is not a fixed factor but is constantly changing in response to environmental factors and life experiences throughout one’s lifespan, even if the brain has already sustained damage [73]. Valenzuela and Sachdev agree with this contention and state that that is why education received in childhood and early adulthood can hypothetically increase the CR volume and, as a result, delay the clinical manifestations of neurodegeneration [74].
Some researchers believe that the mechanisms underlying the BCR are triggered in the selective strengthening and recruitment of neuronal connections. The results of a meta-analysis show that BCR preserves cognitive abilities and executive function despite the decrease in the volume of the hippocampus and the associative frontoparietal cortex due to progressive neurodegeneration [75].
Erratic functioning of brain regions (brain network) is one of the marked features of neurodegeneration. Compensatory BCR function allows one to reorganize brain network activity and preserve cognitive functions [76].
Therefore, the BCR functions differ between healthy individuals and patients with neurodegenerative disorders. In normal aging, an effectively functioning BCR ensures a balance of brain network activity and energy saving during intellectual tasks [77]. At the symptomatic disease stages, excessive interneuronal activity, reflecting the hypercompensatory function of the reserve, contributes to the accelerated depletion of brain structures, promoting the development of clinical and psychopathological manifestations of AD [20, 78].
Prospects for further research on the brain and cognitive reserve
The concept of BRC as a dynamic system retaining the ability to change under the influence of environmental factors and life experiences throughout life even in the presence of neurodegeneration is consistent with the results of meta-analyses, which showed the efficacy of neurocognitive training for brain resilience augmentation even in patients with MCI and AD [79]. A review of neuroimaging studies showed that elderly patients with AD and amnestic MCI retain compensatory mechanisms of neural network activation during cognitive tasks [80]. The authors of other studies define BCR as the ability to form effective and flexible cognitive strategies, which can be augmented through neurocognitive interventions, implying a potential role in AD dementia prevention programs [81, 82].
Thus, promising research directions in the BCR concept development are as follows:
- determination of the reserve volume associated with the degree of brain resilience to neurodegeneration; that is, the transition of AD from the latent to the clinical stage and
- alignment of reserve parameters with different biomarkers (biomarkers of neurodegeneration and disease progression) to predict the probable relationship between pathological and protective factors. This is necessary for the development of individual programs aimed at preventing the transition of preclinical AD stages to clinical ones [77, 78].
Limitations
The holistic nature of the scientific publication coverage achieved with the combination of selected keywords means that this review includes all existing scientific papers on the given topic from the PubMed database and the eLIBRARY electronic library. A limitation with the inclusion of some publications in this review was their descriptive nature. The search was also constrained by the above-mentioned search engines and keywords. In our opinion, a common drawback of the publications included in this review is the heterogeneity of the study materials (laboratory animals, humans) in the studies included in meta-analyses; the retrospective nature of the meta-analyses; and the checkered nature of the studies that were initially included in the meta-analysis. Therefore, the authors of this review admit the limitations of the information presented herein.
This review may be of interest to specialists in the field of mental health and cognitive neuroscience.
Conclusion
The authors of this study believe that the mechanisms of AD pathogenesis cannot be properly understood without taking into account the interactions between pathogenic factors and brain repair mechanisms, such as the BCR. BCR slows the rate of transition from preclinical to clinical disease, thereby changing the AD prognosis. The BCR concept allows one to shift the emphasis towards the prevention of preclinical AD and augment therapeutic efforts at the symptomatic stages of the disease by maintaining and enhancing compensatory mechanisms. In this work, we described the different positions held by researchers regarding the BCR mechanisms and functions at different stages of AD. According to some researchers, interventions aimed at mechanisms of AD pathogenesis and the possibility of their regulation would reduce age-related morbidity and promote healthy aging. The BCR concept actualizes the problem of searching for compensatory strategies for AD-associated cognitive deficit, assessing the structure and volume of the reserve, developing and implementing programs for its maintenance, and battling its depletion as early as in the preclinical stage of the disease.