INTRODUCTION
The number of people who are dependent on psychoactive substances is rapidly increasing worldwide, including in Russia [1, 2]. According to the World Health Organization (WHO), alcohol and drug abuse, as well as the use of other psychoactive substances, has become an epidemic in this early 21st century [3, 4]. It should also be noted that the number of affected families by addiction and requiring professional and timely assistance is also on the increase [5]. Differences amongst individuals in the propensity for addictive behavior, including nicotine dependence, are partially mediated by genetic factors [6]. Current estimates of heritability for all major addictive disorders range from 40% to 80% [7].
Addictive behavior is one form of deviant behavior that arises from the desire to escape reality [8]. The presence of addictive behavior indicates impaired ability to adapt to altered environmental conditions [9]. Addictive behavior traditionally includes alcohol abuse, toxicomania, drug addiction, tobacco smoking (chemical dependencies), as well as computer addiction, gambling, love addictions, sexual addictions, workaholism, and food addiction (overeating, fasting) [10]. Disorders related to psychoactive substance use represent the most common and severe forms of addiction, classified under the International Classification of Diseases, 10th Revision (ICD-10), code F1: “Mental and behavioral disorders due to psychoactive substance use” [11].
Such functions as mood, emotions, cognition, motor abilities, and circadian and neuroendocrine rhythms — including appetite, sleep, and reproductive activity — are regulated by the serotonin system in the midbrain [12]. Fluctuations in serotonin levels is one of the effects of addictive behavior, underscoring the importance of the genes that encode serotonergic receptors and the transporters in the pathogenesis of dependence [13]. One of the candidate genes that affect the development of dependences is the SLC6A4 serotonin transporter gene [10]. Recent studies have demonstrated that the 5-HTTLPR (serotonin transporter-linked polymorphic region) variant in this gene is associated with smoking behavior; however, the level of its implication remains inconclusive due to insufficient research [10, 14].
Studies of the 5-HTTLPR pathological allele in the SLC6A4 gene indicate that there is a connection between various mental disorders and the transcriptional activity levels of the S and L-alleles [15]. For example, reduced activity of the S-allele has been associated with anxiety, depression, suicide attempts, and bipolar disorder, whereas enhanced activity of the L-allele is considered protective against depression, but has also been linked to suicidal behavior, nicotine dependence, and attention-deficit/hyperactivity disorder [15–17]. The aforementioned alleles may also influence treatment efficacy; for example, serotonin reuptake inhibitors may prove more effective in patients with depression and posttraumatic stress disorder who carry the L-alleles [18]. In particular, S-allele carriage is associated with an increased risk of adverse outcomes as relates to alcohol use, mediated by reduced sensitivity to ethanol [19].
The aim of this study is to assess the role of 5-HTTLPR variations in the SLC6A4 gene of the serotonergic system in the development of addictive disorders.
METHODS
Eligibility criteria
Inclusion criteria:
- original research and meta-analyses regarding the role of the 5-HTTLPR variant in the SLC6A4 gene in the development of addictive disorders, including interaction of genetic and environmental factors;
- publications analyzing the pharmacogenetic aspects of the use of antidepressants (selective serotonin reuptake inhibitors, SSRIs) in carriers of different 5-HTTLPR polymorphisms;
- studies related to ethnic differences in the S and L-alleles distribution and their association with clinical outcomes.
Exclusion criteria:
- case reports and case series without the use of control groups;
- publications related solely to therapy for addictive disorders without the analysis of genetic factors;
- publications in languages other than Russian or English.
Information sources
The search was conducted in the electronic databases MEDLINE and eLIBRARY.RU. The search was carried out in December 2024.
The search period covered ran from January 2003 to December 2024. The search was limited to 2003 because that year marked the publication of the first fundamental studies on the role of 5-HTTLPR [20], which laid the foundation for the study of the interaction between this polymorphism and mental disorders, as well as addictive behavior.
Search strategy
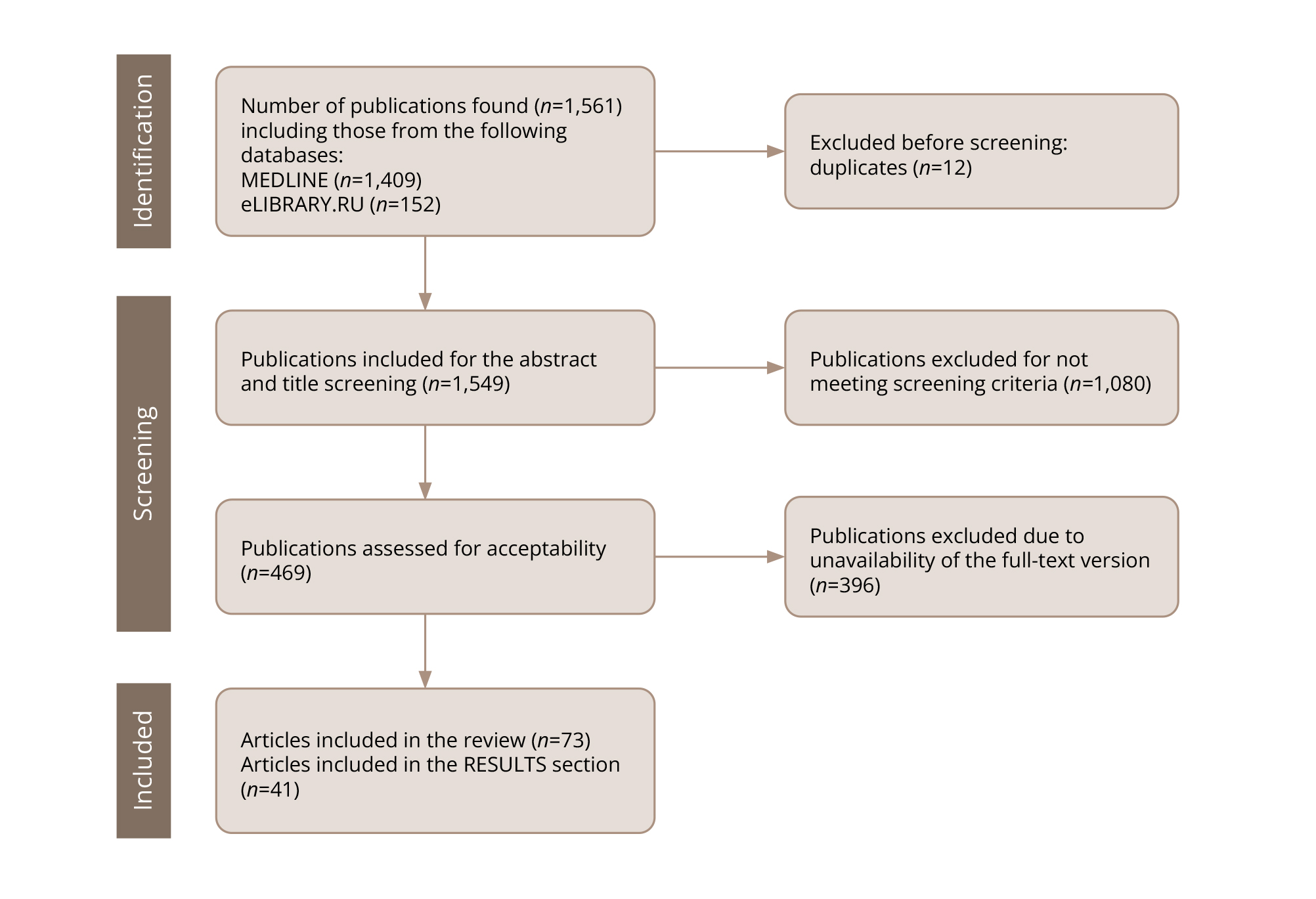
The following combination of keywords in Russian and English were used to search for publications: “SLC6A4”, “5-HTTLPR”, “addictive disorders”, “pharmacogenetics”, “serotonin”, “antidepressants”, “ethnic differences”. The search for publications was performed in stages. The search sequence is shown in Figure 1.
Selection process
Each publication was identified by a manual search. Several specialists from the group of authors of this article conducted the search and selection of publications (see Authors' contributions section). Some publications selected at the screening stage were excluded from further analysis once it became clear that they did not meet the eligibility criteria (Figure 1).
Analysis of the results
The authors analyzed each publication and summarized information from the selected sources. The results of the summarization are presented in the structured text and figures.
RESULTS
The SLC6A4 gene and its relation to psychiatric peculiarities
The 5-HTTLPR (rs4795541) polymorphic region is a functional insertion/deletion polymorphism of 44 base pairs in the promoter region of the SLC6A4 serotonin transporter gene (Figure 2) [21]. 5-HTTLPR is one of the variants that has most extensively been studied in patients with mental disorders [22–24]. It has also been widely investigated in the context of intermediate phenotypes, such as neuroimaging modalities and gene-environment interactions, with the latter typically examined in relation to affective and anxiety phenotypes [21, 25, 26].
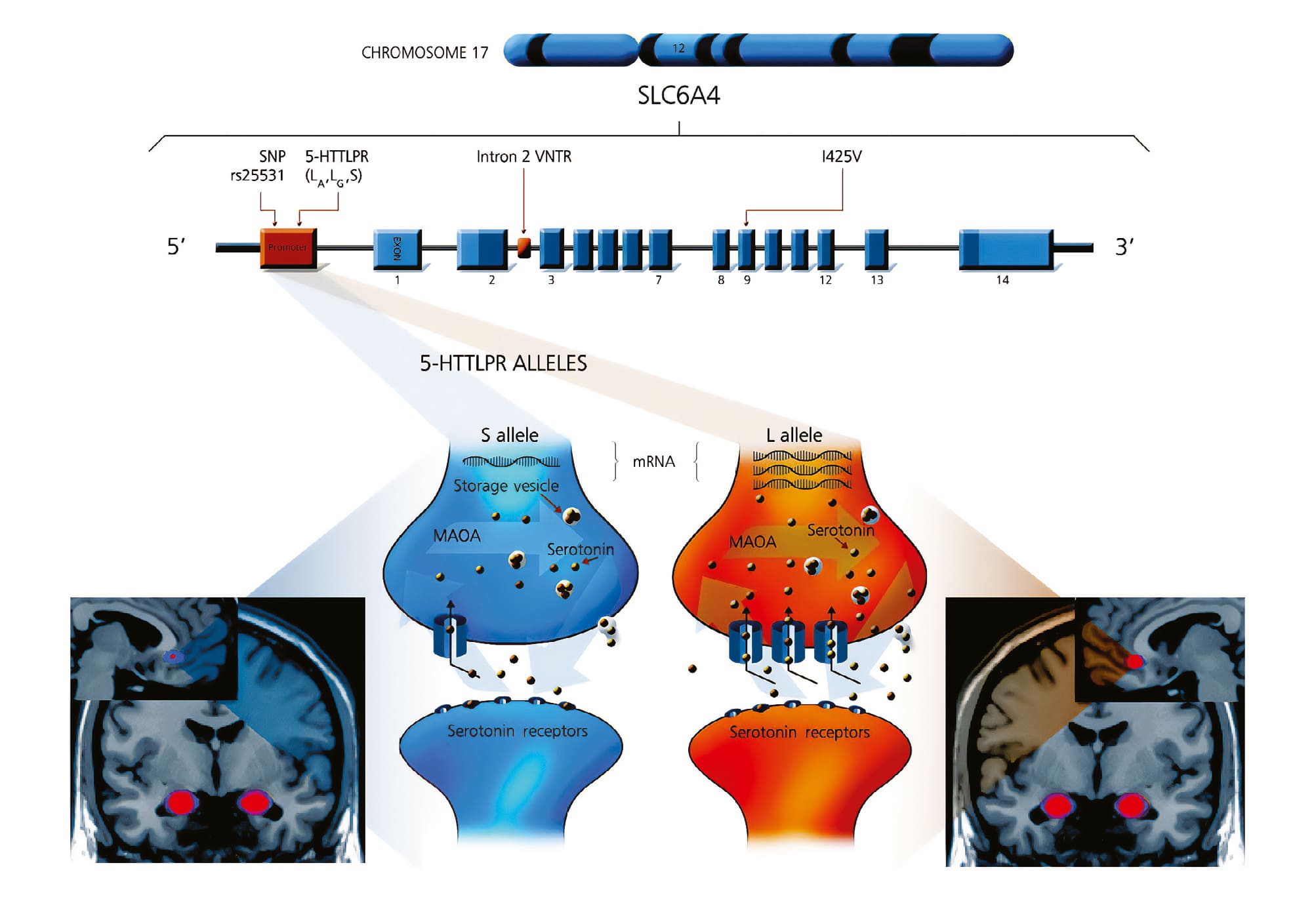
Figure 2. Mapped illustration of the 5-HTTLPR variant in the SLC6A4 gene with allele variants.
Note: 5-HTTLPR — serotonin transporter-linked polymorphic region; CHROMOSOME 17 — the 17th human chromosome, which contains the SLC6A4 gene; Intron 2 VNTR — variable number tandem repeat); МАОА — monoamine oxidase A; mRNA — messenger RNA; rs25531 — is the identifier for a single nucleotide polymorphism (SNP) in the SLC6A4 gene, which affects the expression of the transporter; SNP — single nucleotide polymorphism.
Source: Gerretsen et al., 2009 [21].
In a study comparing the frequency of pathogenic variations in 5-HTTLPR and rs25531 A<G of the SLC6A4 gene among the Yakuts and other population samples, a high frequency of the S-allele was identified, which was similar to that observed in Chinese and Japanese populations [14, 25]. According to the study by Nardi et al., the S-allele (deletion) is associated with a lower expression of the serotonin transporter gene [28]. Moreover, carriers of the S-allele demonstrate increased sensitivity to environmental stimuli [28], which likely contributes to the accumulation of this allele among the Yakuts [14].
Some mental disorders with a comprehensive mechanism of pathogenesis (such as schizophrenia) are associated with a disruption of the serotonin system, which affects the development and differentiation of neurons [29]. Moreover, its transporter, encoded by the SLC6A4 gene, plays a key role in the regulation of the activity level of the serotonergic system [30].
It has been reported that there is a link between altered DNA methylation of the gene encoding the serotonin transporter SLC6A4 and mood disorders, anxiety, as well as amygdala responsiveness [31]. Furthermore, some studies have evaluated the epigenetic changes in the SLC6A4 gene in schizophrenia patients [32–34]. CpG sites (DNA regions consisting of cytosine and guanine separated by a phosphate) of the SLC6A4 gene are known to exhibit changes in methylation levels in patients with bipolar disorder [35]. Male patients with schizophrenia also demonstrated similar results [36].
The impact of the 5-HTTLPR variant of the SLC6A4 gene in the development of addictions
Scientific studies in psychogenetics over the past decade have demonstrated that a significant number of mental disorders have a genetic origin [37]. It should be noted that alcohol abuse is the leading cause of disability and mortality amongst people [38]. The lack of awareness about the harmful effects of alcohol and commitment in society to the ritual of merrymaking, where alcohol is a key element in bringing young people together, can lead to the emergence of behavioral patterns of alcohol consumption [39].
There are two types of addictive disorders:
- chemical addictions (alcohol abuse, drug addiction, toxicomania, etc.);
- non-chemical addictions (pathological gambling, computer addiction, Internet addiction, etc.).
In combination, they can lead to organic disruptions in the higher nervous functions, which can ultimately result in the development of mental disorders [40, 41]. Data suggest that there may be differential genetic vulnerability to alcohol abuse and opiate dependence in serotoninergic genes [42]. There is also evidence that the serotonin system plays a role in the pathogenesis of multiple neuropsychiatric disorders and may be involved in addictions such as smoking, since nicotine increases serotonin production in the brain [43–45]. It is assumed that nicotine and other components of tobacco smoke may contain serotonin and thereby contribute to the development of homeostatic resistance [46]. According to some researchers, the genetic variants of different nations lead to different patterns. For example, among residents of Texas (USA) with the LL genotype, smoking was more common than among carriers of the S-allele [47], whereas the 5-HTTLPR variant of the serotonin transporter gene and any association with smoking have not been documented among the Polish population [48].
It is well known that the genetic basis of alcohol abuse lies in the mechanism of ethanol metabolism and the reward system (the neurobiological system associated with dopamine production and the development of addiction) [49]. The scientific community has shown a greater interest in the association between changes in the promoter region of the serotonin transporter gene SLC6A4 and alcoholism [50]. The S-allele is associated with alcohol consumption, while the L-allele is associated with a positive pharmacological response during the resolution of the withdrawal syndrome [51, 52].
The effects of the 5-HTTLPR variant of the SLC6A4 gene on the outcomes of therapy with antidepressants in various ethnic groups
SSRIs (citalopram, escitalopram, fluoxetine, fluvoxamine) and serotonin modulators with SSRI-like properties are the main pharmacological options for treating major depressive and anxiety disorders [53–55]. The updated guidelines of the Clinical Pharmacogenetics Implementation Consortium (CPIC) emphasize the import of genotyping CYP (CYP2D6, CYP2C19, CYP2B6) genes for dosage optimization; however, our knowledge on the pharmacodynamic SLC6A4 gene remains insufficient for clinical application [56, 57]. Antidepressants constitute the main therapeutic option for patients with depression; however, about 50% of patients fail to achieve an adequate response to them [58]. The site of action of SSRIs is the serotonin transporter, which means that the concentration of this protein can affect its efficacy both directly and through adaptive changes in the serotonergic function [59, 60]. Due to the differences in the transcriptional activity of 5-HTTLPR, the dose of SSRIs may inhibit a greater proportion of serotonin in individuals carrying the S-allele, leading to a rapid accumulation of synaptic serotonin and increasing the risk of adverse reactions [20]. The Biallelic (5-HTTLPR) and triallelic (5-HTTLPR/rs25531) patterns in the SLC6A4 gene are frequently studied, but any idea of association with the antidepressant response remains tenuous [61]. Researchers note differences in the response to SSRIs depending on ethnic variations in 5-HTTLPR: the S-allele is associated with a better antidepressant response in Koreans and Japanese, while the L-allele is associated with a better response in Europeans. However, it is unclear whether the 5-HTTLPR variant and its high expression variant rs25531 have any association with the response to antidepressants [62].
DISCUSSION
Brief interpretation of the results
The 5-HTTLPR variant of the SLC6A4 gene may interact with the environment and affect the development of addictive disorders [59, 60]. Such stressful events as losses or unfavorable household conditions may have a greater effect on patients with the S-allele, making them predisposed to addictive behavior [63]. It has also been shown that the presence of the S-allele may lead to a reduction in serotonin concentration in synapses, which, in turn, is associated with an increased predisposition to the development of mental disorders and addictive behavior [63, 64]. At a physiological level, this may manifest as emotional instability and increased sensitivity to stress [15, 65]. This emphasizes the importance of considering both genetic and environmental factors when assessing the risk of developing dependences [61, 66]. As can be seen in Table 1, the distribution of genotypes (LL, SL, SS) and alleles (L/S) of the 5-HTTLPR variant of the SLC6A4 gene varies greatly across different ethnic groups. For instance, in Asian populations (Japanese, Chinese, Yakut), the S-allele predominates (70.6%–80.9%), whereas in European populations (Russian, Ukrainian, Belarusian), the L-allele is more frequently encountered (38.5%–66.7%) [26]. These differences indicate the need to consider population specificity when analyzing genetic risks [26]. Fundamental studies have not identified any unique neurobiological markers (e.g., features of neuroimaging or immune parameters) that would clearly distinguish carriers of the S-allele from patients with other genetic profiles [20].
Table 1. The frequencies of the genotypes and alleles of the 5-HTTLPR variant in the SLC6A4 gene in various populations [26]
|
Population |
n |
Frequency of genotypes, % (n) |
Frequency of alleles (%) |
Reference |
|||
|
LL |
SL |
SS |
L |
S |
|||
|
Russian (St. Petersburg) |
908 |
38.10 (346) |
46.69 (424) |
15.19 (138) |
61.5 |
38.5 |
[67] |
|
Ukrainian |
60 |
21.21 (14) |
37.87 (25) |
40.90 (27) |
61.5 |
38.5 |
|
|
Belarusian |
39 |
46.15 (18) |
41.02 (16) |
12.82 (5) |
66.7 |
33.3 |
|
|
Chuvash |
372 |
24.46 (91) |
51.61 (192) |
23.92 (89) |
50.3 |
49.7 |
|
|
Kabardian |
289 |
26.64 (77) |
44.63 (129) |
28.71 (83) |
49.0 |
51.0 |
|
|
Tatar |
142 |
26.05 (37) |
51.40 (73) |
22.53 (32) |
51.8 |
48.2 |
|
|
Yakut |
158 |
5.7 (9) |
32.3 (51) |
62.0 (98) |
21.8 |
78.2 |
[26] |
|
Chinese (Beijing) |
558 |
6.09 (34) |
36.02 (201) |
57.88 (323) |
24.1 |
75.9 |
[44] |
|
Thai |
187 |
9.09 (17) |
36.89 (69) |
54.01 (101) |
27.5 |
72.5 |
[20] |
|
Taiwanese |
192 |
10.93 (21) |
36.97 (71) |
52.08 (100) |
29.4 |
70.6 |
[68] |
|
Japanese |
101 |
3.7 (4) |
31.4 (31) |
65.7 (66) |
19.3 |
80.7 |
[69] |
|
Japanese (Tottori) |
501 |
3.19 (16) |
31.73 (159) |
65.06 (326) |
19.1 |
80.9 |
[70] |
|
Chines (Shanghai) |
587 |
6.30 (37) |
41.39 (243) |
52.29 (307) |
27.0 |
73.0 |
[71] |
Note: The specified samples (Russian — St. Petersburg, Chinese — Beijing, Japanese — Tottori, Chinese — Shanghai) are consistent with the data of original studies (see the references in the table) and reflect local rather than general national samples.
Discussion of the results
Recently, in the study by Bousman et al., the authors excluded the SLC6A4 gene from clinical recommendations due to conflicting data and insufficient evidence for its clinical implementation [56]. However, in their systematic review and meta-analysis, Stein et al. showed that the pathological variant of 5-HTTLPR can serve as a marker for antidepressant treatment outcomes in patients with mental disorders and may be particularly relevant for the use of SSRIs in individuals of European descent [68]. Laje et al. [69] and Rahikainen et al. [70] demonstrated that male patients with a low-functioning genotype SS 5-HTTLPR/rs25531, who were on SSRIs (citalopram), were at increased risk of violent suicide (bringing to suicide). At the same time, studies conducted in Korean patients with severe depression by Jang et al. showed that carriers of the ss 5-HTTLPR genotypes had significantly better treatment outcomes, while the genotype containing the g (ag+gg) rs25531 variant was associated with remission only [71]. Despite the fact that this pathological allele is involved in the development of addictive disorders, it cannot serve as a clinical marker due to a lack of evidence. Moreover, at the current stage of research, many investigators associate this genetic variant with other mental disorders, such as depression and anxiety (Table 2) [72, 73].
Table 2. Studies of 5-HTTLPR variations of the SLC6A4 gene
|
Category |
Brief description |
References |
|
Human studies |
||
|
Mental disorders |
Relation to schizophrenia, depression, and anxiety in various populations. |
|
|
Smoking/nicotine |
Association with nicotine dependence and behavioral patterns. |
|
|
Alcohol |
The role of 5-HTTLPR in the development of alcohol abuse. |
|
|
Anxiety/stress |
Association with panic attacks and stress reactivity. |
|
|
Pharmacokinetics |
Effects on the efficacy of antidepressants (SSRIs). |
|
|
Personality/neurodegeneration |
Role in personality traits and neurodegenerative processes. |
|
|
Population differences |
Ethnic variability of alleles and risks. |
|
|
Epigenetics |
Promotor hypermethylation and its clinical correlates. |
|
|
Animal studies |
||
|
Epigenetics/environment |
The effect of environmental enrichment on SLC6A4 expression and demethylation in mice. |
[34] |
Limitations
Although the coverage of scientific publications based on the keywords used in MEDLINE and eLIBRARY.RU could be considered comprehensive, the descriptive nature of some publications prevented us from including them in the study. The limitation of the search by the specified search engines and keywords led to the heterogeneity of the study material in the meta-analyses, as well as to the retrospective nature of the meta-analyses themselves and the insufficient comprehensiveness of the studies initially selected for them. In this review, only one gene SLC6A4 and its two variants (5-HTTLPR and rs25531 A<G) were considered. Furthermore, since the pleiotropic effects of the SLC6A4 gene are associated with depression and anxiety, this limits the possibility of isolated interpretation of its role in the development of addictive disorders. The authors acknowledge the limitations of the information presented and recognize that, even with the most thorough possible approach, the study cannot encompass all aspects of the topic being considered.
CONCLUSION
This review attempted to systematize the data on the role of the 5-HTTLPR variant of the SLC6A4 gene in the development of addictive disorders, highlighting its ambiguous nature and pleiotropic effects. In contrast to previous studies, the emphasis here is centered on the need for a multidimensional approach to risk assessment that takes into account genetic, environmental, and ethnic factors. Further studies with an in-depth analysis of the molecular mechanisms of the interaction between the 5-HTTLPR variant of the SLC6A4 gene and the serotonergic system are needed. Future research should also include the development of personalized prevention and treatment strategies, which can potentially improve the efficacy of addiction treatment and reduce the frequency of adverse reactions.
Authors’ contribution: Alexey Krylov — concept development, analysis and interpretation of the obtained data, and writing the manuscript. Nadezhda Pavlova, Alexey Bochurov — concept development, manuscript editing, data collection, and analysis.
Conflict of interest: The authors declare no conflicts of interest.