The article took part in the competition of scientific papers of early-career psychiatrists.
INTRODUCTION
Cognitive impairment is one of the main factors that disrupt daily social functioning and quality of life. This issue has become more acute in the context of the coronavirus (COVID-19) pandemic. The symptoms of cognitive impairment were noted not only in patients with the acute form of COVID-19 but also in persons with an asymptomatic course of the disease and in patients six months after recovery [1, 2]. The following symptoms were commonly observed: headache, dizziness, loss of sense of smell and taste, an increased sense of anxiety, agitation, impaired attention and confusion [3], delirium [4–6], inflammatory complications (meningitis, encephalitis, encephalomyelitis) [7] and stroke [8, 9]. Furthermore, in a recent meta-analysis J. Deng et al. determined that depression was observed among 45% of the patients with COVID-19, anxiety in 47%, and insomnia in 34% of patients [10]. These rates significantly exceed the levels of depression, anxiety, and insomnia before the pandemic among both hospital patients and the general population. Studies also report that mental disorders tend to be more common among hospitalised patients with COVID-19 [11], and clinically significant cognitive decline is especially frequent among elderly people who have been infected with this virus [12]. Although it is difficult to correctly assess the symptoms of cognitive impairment during the acute phase of COVID-19, the Mini Mental State Examination (MMSE) detected the presence of such symptoms among 33% of patients [13]. According to the systematic review findings, the majority of patients with COVID-19-related severe acute respiratory syndrome (SARS) or Middle East respiratory syndrome (MERS) do not develop mental disorders [15]. However, 34% of patients tend to show signs of memory disorders [15].
The existing literature suggests that about one-third of patients have cognitive impairment symptoms upon discharge from the hospital. Psychopathological symptoms often remain present longer than complications associated with the respiratory or cardiovascular systems [15, 16]. An online survey in the United States showed that among 1,500 people infected with COVID-19, about 50% of respondents had difficulties in concentrating on a task for a long time [15]. It was determined that the severity of cognitive impairment during the post-COVID-19 syndrome directly correlates with the severity of the disease. The severity of the COVID-19 course depends on the level of hypoxemia, increased concentration of D-dimer in the acute period, residual pulmonary insufficiency, the level of C-reactive protein (CRP), and increased density of the white matter of the brain [17]. Besides the COVID-19 itself, various psychiatric disorders were caused by contradictory and often alarming opinions about the pandemic expressed by the medical community and media representatives [18].
Although diagnosis and treatment of mental disorders and cognitive impairment during the acute period of COVID-19 are not prioritised due to the urgency of somatic or neurological pathology, they may cause difficulties in the long-term perspective. Taking this into account, we conducted an exploratory study of factors that may be associated with cognitive decline during the COVID-19 pandemic among the polyclinic patients of the North-West Region of Russia. The objectives of the research were as follows: 1) to assess the prevalence of cognitive decline complaints in patients diagnosed with COVID-19; 2) to objectify the presence of cognitive impairment in patients with subjective cognitive decline complaints; 3) to assess the relationship between age and education level of patients with the emergence of objective and subjective cognitive decline complaints; 4) to assess the role of the severity of COVID-19 on cognitive status.
METHODS
Study design
The cross-sectional multicentre observational study was conducted in the polyclinic unit of Saint Petersburg and regions of the North-Western Federal Region.
Procedure
Within the framework of mandatory practical training, resident doctors of the V.M. Bekhterev National Medical Research Centre for Psychiatry and Neurology collected anamnesis and laboratory parameters of polyclinic patients, including the nasopharyngeal polymerase chain reaction (PCR) test, C-reactive protein (mg/L), platelets (109/L), and lymphocytes (109/L). Further, a physical examination was performed and included measurement of respiration rate, oxygen saturation in the blood (SpO2 (%), and computed tomography (CT) of the chest (volume of lesion) according to the recommended criteria (CT-1 — Minimal (<25% of volume), CT-2 — Average (25–50% of volume), CT-3 — Significant (50–75% of volume), CT-4 — Subtotal [19]). Due to the non-interventional nature of the study, investigators collected only those laboratory parameters, which were available for patients who agreed to participate in the study. All study participants were screened for the presence of anxiety and depression, using the Hospital Anxiety and Depression Scale (HADS) [20]. Furthermore, participants with cognitive decline complaints were screened for the presence of cognitive impairment using Montreal Cognitive Assessment (MoCA) [21, 22].
Prior to the study, the Young Scientists Council of the V.M. Bekhterev National Medical Research Centre for Psychiatry and Neurology instructed residents on the methodology of conducting an assessment using MoCA and HADS and provided step-by-step video description of the required scope of the survey.
Instruments
HADS is one of the most commonly used scales for assessing anxiety and depression among patients in a general hospital setting [20, 23]. It is a 4-point Likert scale, consisting of two subscales with seven items each: first subscale measures anxiety (HADS-A) and second subscale measures depression (HADS-D). The total score is obtained by summarising the scores within each subscale. The scores from 0 to 7 represent “normal,” 8–10 “mild,” 11–14 “moderate,” and 15–21 “severe” levels of anxiety and depression according to international guidance [20].
MoCA is a screening test used to detect mild cognitive impairment (MCI) at a very early stage, which makes it possible to diagnose and treat patients faster. MoCA includes nine areas: executive function, fluency, orientation, computation, abstraction, delayed recall, visual perception, naming, and attention. A score less than or equal to 26 points out of 30 indicates cognitive impairment [2, 12, 24].
Participants
Inclusion criteria:
1) patients from 18 to 70 years old who have given voluntary informed consent to participate in the study and who were able to understand tasks of the research scales;
2) patients who received primary health care (in a polyclinic unit) in St. Petersburg and regions of the North-Western Federal District of Russia;
3) patients receiving primary health care from residents of the V.M. Bekhterev National Medical Research Centre for Psychiatry and Neurology.
Exclusion criteria:
1) patients in need of emergency hospitalisation at the time of applying for outpatient primary health care and specialised medical care due to the deterioration of their general condition;
2) patients in need for emergency, resuscitation or other medical care during hospitalisation due to the severity of their current condition;
3) refusal of the patient to participate in the study at any stage;
4) aggravation of the patient’s mental or somatic condition at any stage of the research.
Statistical analysis
For the purposes of this study, subjective cognitive decline is defined as a self-perceived cognitive decline in cognitively normal people [31]. In line with this definition the sample was divided in two subgroups: those who complained about decreased cognitive functions and those who had no cognitive complaints. Based on the laboratory and instrumental examination, participants were divided into three groups depending on the severity of their condition: severe, moderate and mild. The criteria for severe degree were as follows: respiration rate >30, SpO2 ≤93% and CT signs typical for a viral lesion (the volume of the lesion is significant or subtotal; CT 3–4). The criteria for moderate degree were as follows: 22 < respiration rate < 30, 95% < SpO2 ≤ 93%, CT signs typical for a viral lesion (the volume of the lesion is minimal or average; CT 1–2), serum CRP >10 mg/l. Mild severity group included persons who had no signs of moderate or severe condition. The severity criteria were determined according to the temporary guidelines for prevention, diagnosis, and treatment of the COVID-19 infection at the time of the study [25].
Mathematical and statistical data processing was carried out using the software product SPSS (v.26.0.0.0) and the programming language R (version 4.0.2) in RStudio v1.4.1717. The arithmetic mean and standard deviation — M(σ), as well as the median and interquartile range — Me (Q1–Q3) were used as measures of the central trend. Categorical variables were described by percentages with the reduction of absolute numbers — % (n) and comparison using Pearson’s criterion χ2. The test for the normality of the distribution was carried out according to the Shapiro-Wilk criterion with the correction of the significance by Lilyfors. Due to the abnormality of the distribution of most variables, the Kraskal-Wallis criterion was used for data with ordinal scales, followed by the application of the Mann-Whitney U criterion for pairwise comparison and considering Bonferroni corrections. Spearman’s criterion (ρ) was used for the correlation analysis.
Ethical approval
The study was conducted in compliance with the current legislation of the Russian Federation, the Helsinki Declaration of Human Rights Protection, and the rules for organising research protocols. Data on the results of invasive research methods were used from available medical documents, if the patient had them at the time of his inclusion in the study. The participants (patients) were informed in full and accessible form about the nature and purpose of the study and provided their written consent. The study was approved by the Local Ethics Committee (IRB Registration number: EC-I-132/20).
RESULTS
Overall sample characteristics
The study included 515 participants, 60% (n=310) of which were women. The mean age of respondents was 41.5 (±15) years (M(σ) for women=40.97 (±15.09) and M(σ) men=42.36 (±14.94)). Overall, 57.6% (n=297) of the participants had higher education, 9.9% (n=51) did not complete higher education, 15.7% (n=81) of respondents had professional, 14.9% (n=77) had secondary education and 1.74% (n=9) of the participants had an academic degree. In total, 74.2% (n=382) of participants were diagnosed COVID-19. In particular, 52.4% (n=270) of patients had instrumentally confirmed COVID-19 infection: 43.1% (n=222) of patients were diagnosed based on the PCR-test results at the time of inclusion in the study, whereas 9.3% (n=48) of patients were diagnosed according to the detected signs of the chest CT. Other 21.7% (n=112) of patients had signs of severe acute respiratory syndrome (SARS) and were diagnosed with COVID-19 (according to medical documents) although PCR test verification of diagnose was not presented.
Prevalence and factors associated with subjective cognitive decline
The overall sample (n=515) was divided into those who complained of subjective cognitive decline (28.5% (n=147)) and those who did not have cognitive complaints (71.4% (n=368)). The groups differed by age, education level, and COVID-19 status. The characteristics of the sample are presented in Table 1.
Table 1. The characteristics of the sample
|
Factors |
Group with subjective cognitive decline (n=147) |
Group without complaints of cognitive decline (n=368) |
χ2 (Pearson’s criterion) |
p-value |
|
Age, Me (Q1-Q3) |
45 (31–62) |
36.5 (29–48) |
111.156 |
0.000 |
|
Male, n (%) |
60 (41%) |
145 (39.4%) |
0.088 |
0.767 |
|
Female, n (%) |
86 (59%) |
223 (60.6%) |
||
|
Education level |
||||
|
Academic degree, n (%) |
0 |
9 (2.4%) |
18.107 |
0.001 |
|
Higher education, n (%) |
74 (50%) |
223 (60.5%) |
||
|
Incomplete higher education, n (%) |
10 (6.8%) |
41 (11.1%) |
||
|
Secondary vocational education, n (%) |
33 (22.4%) |
44 (11.9%) |
||
|
Secondary education, n (%) |
30 (20.4%) |
51 (13.8%) |
||
|
COVID-19 positive status, n (%) |
116 (78.9%) |
266 (72.3%) |
20.748 |
0.000 |
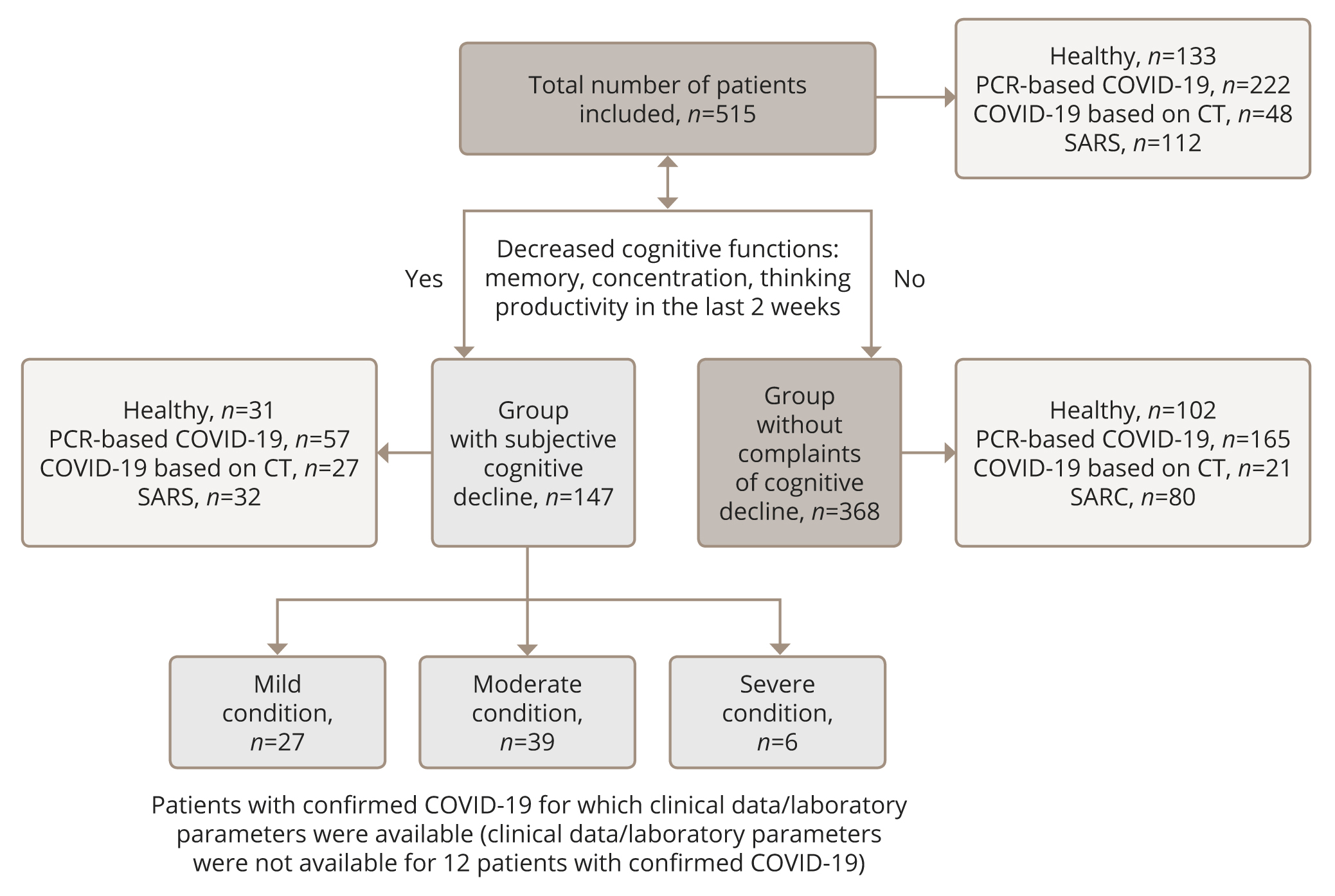
In the group of patients with subjective cognitive decline, 116 patients were diagnosed with COVID-19 or showed signs of SARS. The complete body of clinical and laboratory data required to determine the severity of the disease was available for 72 patients. Thus, a severe course of the disease was found in six people; the average condition — in 39 patients; and mild — in 27. The sample stratification is illustrated in Figure 1.
Figure 1. Flow chart of the study
Out of 515 participants, HADS was completed by 503 participants. The distribution of anxiety and depression levels significantly differed in the groups with or without complaints of subjective cognitive decline (Table 2).
Table 2. Comparative analysis of the HADS indicators among patients with and without complaints of subjective cognitive decline
|
Scale |
Median and interquartile range, Me (Q1–Q3) |
|||
|
HADS |
With subjective cognitive decline (n=147) |
Without complaints of cognitive decline (n=357) |
U |
p-value |
|
HADS-A |
9 (5–12) |
4 (1–8) |
36895.5 |
0.000 |
|
HADS-D |
7 (4–11) |
3 (1–27) |
3739.0 |
0.000 |
Prevalence and factors associated with objective cognitive decline in COVID-19 patients
Patients with subjective cognitive complaints were given an opportunity to undergo a screening examination of the cognitive functions using the MoCA test. Out of 147 participants with subjective cognitive decline complains, the MoCA test was performed on 125 (response rate 85%, no incomplete tests) respondents and demonstrated the following results (Table 3). Objective cognitive decline (MoCA score less than 26 points) was detected in 40% (n=50) of participants with subjective cognitive complaints, whereas COVID-19 was diagnosed in 31% (n=39) of participants. The median MoCA score Me (Q1–Q3) was 27 (25–28), indicating the normal range. No significant correlations were found between the MoCA score and the levels of anxiety and depression according to HADS (p >0.05) among those who complained of memory and attention loss. There was no relationship between the overall MoCA score and age according to the nonparametric Spearman correlation test (ρ=-0.193, p=0.099).
Table 3. The results of the MoCA test in a group of patients with subjective complaints of cognitive decline
|
Median and interquartile range, Me (Q1–Q3) |
|
|
MoCA |
Patients with subjective cognitive decline (n=125) |
|
Trail making test |
1 (1-1) |
|
Copy square box |
1 (1-1) |
|
Clock drawing test |
3 (2-3) |
|
Naming |
3 (3-3) |
|
Repetition of digits |
2 (1-2) |
|
Tap hand at letter |
1 (1-1) |
|
Serial account |
3 (2-3) |
|
Repetition of phrases |
2 (2-2) |
|
Verbal fluency |
1 (0-1) |
|
Abstraction |
2 (1-2) |
|
Delayed recall |
4 (3-5) |
|
Orientation |
6 (6-6) |
|
Total MoCA score |
27 (25-28) |
Statistically significant differences were found in MoCA scale subsets between the three groups of patients stratified by the severity of the COVID-19 course (Table 4). In particular, the group of patients with a mild course of the COVID-19 were more successful compared to the patients with a moderate course in the following tasks: clock drawing test, phonetic verbal fluency, and generalising concepts (abstraction). We observed that a moderate course of COVID-19 predominantly affected optic-spatial and regulatory cognitive functions, including a decrease in speech fluency and the level of the abstraction process. Compared to the patients with a mild course of the disease, a group of patients with a severe course coped worse with the task of paying attention to the “serial account”. Since attention is a “cross-cutting” mental process, its disturbance may affect the state of other cognitive functions. Median values in the groups showed an increase in cognitive impairment depending on the severity of COVID-19. Based on these results, we may suggest that cognitive functions are influenced by the severity of the COVID-19 course.
Table 4. Comparative analysis of age and MoCA scale subsets across three groups of patients stratified by the severity of COVID-19 course
|
Median and interquartile range, Me (Q1–Q3) |
|||||
|
MoCA subtest |
Mild condition (n=27) |
Moderate condition (n=39) |
Severe condition (n=6) |
p-level for Kruskal-Wallis test |
p-value for pairwise comparison |
|
Clock drawing test |
2 (2–3) |
3 (3–3) |
3 (2.75–3) |
0.022 |
Mild-Moderate: 0.029 |
|
Verbal fluency |
1 (1–1) |
1 (0–1) |
1 (0–1) |
0.008 |
Mild-Moderate: 0.006 |
|
Abstraction |
2 (2–2) |
2 (1–2) |
2 (1–2) |
0.04 |
Mild-Moderate: 0.035 |
|
Serial account |
3 (2–3) |
2 (2–3) |
2 (0.75–2) |
0.011 |
Mild-Severe: 0.011 |
|
Total MoCA score |
27 (25.5–28) |
25 (24–27.5) |
23.5 (21.75–26) |
0.04 |
Mild-Severe: 0.042 |
|
Age |
40 (32–52) |
53 (40.5–66) |
49 (23–59) |
0.049 |
Mild-Moderate: 0.047 |
DISCUSSION
Main result
This study analysed a cognitive profile of patients diagnosed with COVID-19, as well as the relationship between subjectively perceived cognitive impairment and objective cognitive and emotional screening results. Our study resulted in three main observations. Firstly, patients with subjective complaints of cognitive decline comprised 43% of the subgroup with diagnosed COVID-19. They were older, had lower levels of education, and higher levels of depression and anxiety. Secondly, according to the MoCA test results, objective cognitive decline was observed in 40% of participants with subjective cognitive complaints, whereas COVID-19 was diagnosed among 31% of participants. However, no significant correlations were found between the MoCA scores and the anxiety and depression levels. Finally, patients with mild severity of the COVID-19 were more successful with MoCA subtests than patients with moderate and severe courses of the disease.
Strengths and limitations of the study
To our knowledge, the current research is one of the first studies exploring objective and subjective cognitive decline among patients with confirmed COVID-19 in Russia. Valuable data was obtained regarding the links between objective and subjective cognitive functioning and the affective state of patients. Further implementation of this study should be considered in the light of the following limitations. Firstly, the study employed a cross-sectional design, limiting the possibility for a prospective assessment of cognitive impairment. Secondly, the scales used for the purposes of this study cannot be the sole basis for diagnosis. Thirdly, participants were unevenly represented in the sample sub-groups. Finally, cognitive functions were not screened in patients who did not complain of cognitive decline. This may have influenced the results as awareness of cognitive problems is a characteristic of mild cognitive impairment. Therefore, patients with more pronounced impairment may have not reported the presence of cognitive deficits.
Comparison with the existing literature
Cognitive impairment is a frequent complaint both during the COVID-19 period and in the post-COVID period. Rass et al. [26] revealed cognitive impairment among 23% of patients with COVID-19. According to the observations of Beaud V, et al. [15], a few days after the discharge from ICU significant cognitive impairment was observed in 38% of patients with COVID-19 based on the MoCA scale results [15]. Among them, 61.5% of patients had frontal cortex dysfunction according to the FAB (Frontal Assessment Battery) [15]. Also, the existing research suggests that subacute cognitive impairment (COVID-19-induced encephalopathy) may occur in patients with the mild/moderate course of COVID-19 more than seven days after the onset of the disease [27]. The severity of cognitive impairment, as a rule, correlates with the severity of COVID-19 [11, 26], which is similar to the results of the present study.
In the research by Matos et al., cognitive dysfunction is observed in all patients based on the MoCA test results. Particularly, the decline was observed in phonemic verbal fluency with a median of six words/min (Q1=5.25, Q3=10.75) and visual-spatial skills with a median of four points (Q1=4, Q3=9) [27]. It was suggested that such cognitive dysfunction is mainly caused by the dysregulation associated with the damage to the frontal lobe. Dysregulatory (dysexecutive) syndrome usually includes emotional, motivational and behavioural symptoms, and cognitive deficits [28]. This syndrome was observed among COVID-19 patients and has been reported in several studies [29, 30]. Cognitive impairment can also be observed during an asymptomatic course of the COVID-19. In a study [12] conducted on 93 newly infected and 102 healthy respondents, there was no significant difference in the overall cognitive assessment scores between the two groups. However, COVID-19 patients showed lower scores in comparison with healthy respondents in the domains of visual perception, naming, and fluency. In our study, patients demonstrated the greatest difficulties with the “drawing the clock” subtest, which reflects impairment of optical-spatial and regulatory functions.
Similar to our findings, the existing research suggests that subjectively perceived cognitive impairments may not always correspond with the results of objective assessment using psychometric scales [32–34]. It has been also determined that worries about potential memory/thinking difficulties were present in 24% of the population and were associated with the risk of MCI [34]. Furthermore, it is known that 12.84% of those who had COVID-19 were diagnosed with psychiatric or neurological impairment for the first time after six months of the disease [36]. Therefore, the group of patients with subjective cognitive decline complaints recruited for the purposes of the current study requires further observation.
It is known that cognitive impairments can be associated with anxiety, depression, and traumatic experiences of hospitalisation [37]. This has led to the uncertainty on whether it is subjective cognitive decline that predicts the subsequent development of neurocognitive disorders or they are caused by anxiety symptoms. The subjective cognitive decline has been shown to strongly correlate with anxiety symptoms among older persons [38], which is similar to the findings of the present study. This is especially pertinent given that anxiety has consistently been identified as a key predictor of neurocognitive disorders in several meta-analyses [4, 8, 10]. Thus, exploring relationships between the subjective and objective cognitive consequences of COVID-19 and their association with the quality of life is needed for assessing the cognitive abilities of patients with post-COVID syndrome [17].
Implementation into practice
In accordance with the findings of the current study, the following suggestions are made. Firstly, further studies on the cognitive profile of patients with COVID-19 may need to employ a longitudinal design, allowing one to perform a long-term assessment of the cognitive impairment. Secondly, psychometric scales shall be used to screen for affective syndromes and cognitive decline in clinical settings during the periods of high incidence of COVID-19. Such screenings might help to detect patients who are in a greater risk of developing depression, anxiety disorders, MCI, and dementia. Thirdly, it is important to note that the MMSE (Mini–Mental State Examination) scale, which is widely used in clinical practice, is insufficiently sensitive for vascular cognitive disorders that occur during the COVID-19 [9, 39]. In our opinion, the MoCA test is able to detect early signs of cognitive decline; therefore, it is more suitable for use in clinical practice. Finally, the diagnosis of cognitive decline cannot be made solely based on the results of the screening scales and subjective complaints of patients. It is crucial to follow the diagnostic criteria of the International Classification of the Disease 10/11.
CONCLUSION
According to the obtained results, subjective complaints about cognitive dysfunction in patients of outpatient units during the pandemic are mainly caused by the emotional state rather than the objective decline in cognitive functions. The severity of the disease affects the functioning of the cognitive sphere, including attention, regulatory functions, and speech fluency. Mild and moderate severity of COVID-19 correlates with clinically determined depression. The absence of this relationship in the case of a severe course of COVID-19 is probably caused by the significant somatic decompensation of patients.