INTRODUCTION
Schizoaffective disorder (SAD) is undoubtedly one of the most significant challenges of modern clinical psychiatry, the importance of which is increasing due to the accumulation of a large body of new data and the development of updated classification systems. [1]. Despite the wide application of this diagnostic category, it remains characterized by low diagnostic stability and validity [2]. Debates is ongoing about whether SAD should be classified as a type of schizophrenia, a type of bipolar affective disorder (BAD), an independent diagnostic category, or whether it is better conceptualized along a continuum of varying combinations of psychotic and affective symptoms. A more precise definition of SAD could reduce diagnostic heterogeneity and improve the reliability of the “schizoaffective disorder” category [1].
A survey of 873 specialists from various countries found that the level of diagnostic agreement for the ICD-11 criteria for SAD was moderate (Cohen’s kappa — a measure of inter-rater agreement — was κ=0.38), although this was significantly higher than the inter-rater agreement level for the ICD-10 criteria for SAD (κ=0.27) [3]. Field studies leading up to the DSM-5 revision have reported moderate inter-rater reliability for SAD (κ=0.5), lower than for bipolar disorder type I (κ=0.56) but higher than for schizophrenia (κ=0.46) [4]. A more recent study revealed that the inter-rater reliability of the DSM-5 SAD diagnosis (κ=0.57) is even lower than that for schizophrenia, bipolar disorder, and major depressive disorder, by an average of 19–22%, in a sample of 7912 patients assessed by different raters. This highlights the importance of the re-diagnosis of patients with SAD [5]. Additionally, low diagnostic reliability was encountered in a study of children and adolescent populations (κ=0.27): much lower than that of schizophrenia (κ=0.56) and bipolar disorder (κ=0.64) [6]. A study involving 33 patients using the M.I.N.I. interview (a brief structured diagnostic interview for major psychiatric disorders in DSM-IV and ICD-10) in a psychiatric hospital in Moscow, where patients were initially diagnosed with SAD (F25 in ICD-10), found that in 23 (69.7%) cases the condition aligned with the diagnostic criteria of bipolar disorder, and that only in 10 (30.3%) patients was the diagnosis of SAD confirmed [7].
The aim of this paper is to summarize both the Russian and international literature on the concept of SAD, including its clinical characteristics, cognitive profile, potential biomarkers, and the evolution of the Schizoaffective Disorder category in international classifications, such as the International Classification of Diseases (ICD) 9th, 10th, and 11th revisions, as well as the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5).
METHODS
Eligibility criteria
Inclusion criteria:
- original studies dedicated to the conceptualization, clinical characteristics, cognitive profile, differential diagnosis, comorbidity, and potential biomarkers of SAD, in which SAD or its types were separated into distinct clearly defined groups or subgroups;
- international classifications (ICD-9, ICD-10, ICD-11, DSM-5).
Exclusion criteria:
- case reports;
- case series;
- articles dedicated to the treatment of SAD.
Information sources
The work was carried out from February to August 2023. The search for sources was conducted in the scientific electronic library eLIBRARY and the PubMed database (full-text articles) with a focus on publications in the past 15 years (2008–2023). Also, the three most significant papers on the topic for an earlier period were included, where the division of two main endogenous mental illnesses with the dominance of psychotic and affective disorders is substantiated and the term “schizoaffective disorder” is introduced [8–10].
Search strategy
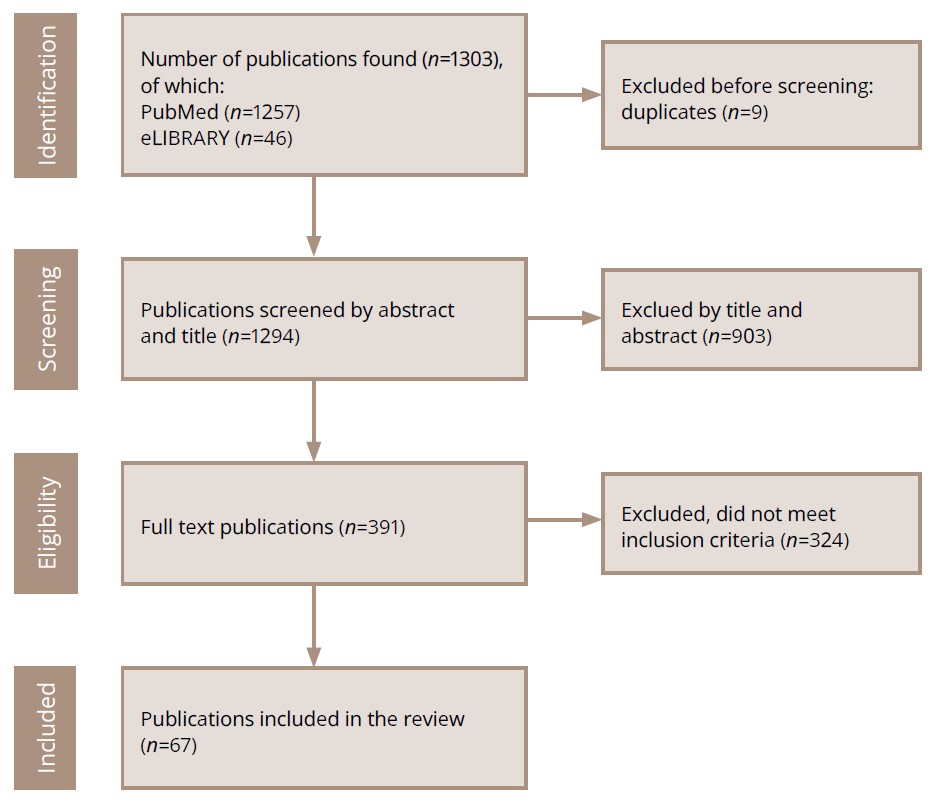
The number of publications in the initial search query by keywords in the PubMed was 1257; in the eLIBRARY — 46. In accordance with the inclusion criteria, 67 publications were selected (including the three works mentioned above [8–10]), of which 56 were in English, 11 were in Russian, including ICD-9, ICD-10, ICD-11 and DSM-5. The number of publications over the past 10 years amounted to 47 (70.1%).
The search strategy for sources is presented in Figure 1.
Figure 1. PRISMA flow diagram of the literature search and the selection process.
Note: Total number of references: 67 +3 most significant works on the designated topic for an earlier period [8–10].
Source: Pavlichenko et al., 2024.
Selection process
The following combinations of keywords in Russian and English were used to select the publications: “schizoaffective disorder”, “concept”, “diagnosis”, “DSM-5”, “ICD-11”, “clinical features”, “comorbidity”, “neuroimaging”, “cognitive impairment”. Keyword searches were also used to find two related words (e.g., “schizoaffective disorder” and “comorbidity”).
Analysis of results
A descriptive approach was applied to summarize the information obtained, which consisted of analyzing and evaluating the data from the point of view of the purpose of the current study.
RESULTS
Schizoaffective disorder in modern disease classifications
In 1899, Emil Kraepelin had the idea to divide the so-called functional psychoses into “dementia praecox” and “manic-depressive insanity” (“manisch-depressives Irresein”) [8]. In later work in 1920, he arrived at the conclusion that it was impossible to draw a satisfactory distinction between the two diseases, although he acknowledged the existence of patients with “irreversible mental decline” [9]. In 1933, the American psychiatrist Kasanin introduced the term “schizoaffective psychosis” based on his study of 9 patients with good premorbid functioning who happened to develop a combination of psychotic and affective symptoms, with full recovery within a few months [10].
The ICD-10 Clinical Descriptions and Diagnostic Guidelines allows for the diagnosis of SAD if the patient’s mental state exhibits “one or, preferably, two typically schizophrenic symptoms (criteria A–D in ICD-10), and two characteristic depressive symptoms (depressive type), manic symptoms such as a prominent elevation of the mood, or a less obvious elevation of the mood combined with increased irritability” (manic type), or “a combination with mixed bipolar affective disorders” (mixed type) [11]. Notably, this version of ICD-10 does not provide a clear specification of episode duration, stating only that schizophrenic and affective symptoms must co-exist “for at least several days”. Meanwhile, the ICD-10 Diagnostic Criteria for Research specifies that the “disorder meets the criteria for one of the moderate or severe affective disorders”, meaning that the duration of depressive and mixed symptoms should be at least two weeks; and manic symptoms, at least one week [12]. Additionally, the state must include one of the typical symptoms of schizophrenia, which, in addition to psychotic symptoms consistent with the schizophrenia criteria in the ICD-10 Clinical Descriptions and Diagnostic Guidelines (criteria A–G), also includes disorganized thinking (“clearly incoherent or irrelevant speech, or frequent use of neologisms”) and catatonia (“frequent occurrence of catatonic behavior, such as posturing, waxy flexibility, and negativism”). The duration of schizophrenia symptoms should be at least two weeks, and within a single episode, they must co-exist with affective symptoms “at least for some time simultaneously” [12].
A diagnosis of “Schizoaffective Disorder” does not apply when schizophrenic symptoms predominate in some episodes and affective symptoms in others, according to the ICD-10 guidelines. In such cases, the diagnosis of two separate disorders is possible when schizoaffective episodes alternate with affective episodes of bipolar or recurrent depressive disorder [11]. On the other hand, if schizophrenic and affective symptoms develop concurrently and are “relatively balanced in severity and duration” in relation to each other, a diagnosis of SAD is in order, even if the schizophrenic symptoms taken separately would justify a diagnosis of “Schizophrenia” [11]. Thus, ICD-10 departs from the hierarchical diagnostic principle that has long prevailed in psychiatry, where psychotic symptoms were considered more significant than affective ones [13]. Notably, in ICD-9, SAD was classified within the schizophrenia group rather than as a separate category [14]. It is also stated therein that the presence of delusions or hallucinations incongruent with mood, including first-rank symptoms, is not sufficient for an SAD diagnosis if these symptoms had not preceded the development of affective syndromes and/or had not persisted after their remission [12].
According to ICD-11, a diagnosis of SAD is warranted when the current episode simultaneously meets the diagnostic criteria for both schizophrenia and a manic, mixed, or moderate-to-severe depressive episode [15]. It is also important to note that in ICD-11, Kurt Schneider’s first-rank symptoms have lost their relevance for the diagnosis of schizophrenia [16]. For schizophrenia, at least one of the following four symptoms is required: “persistent” delusions, “persistent” hallucinations, disorganized thoughts, or passivity and delusions of control [15].
A schizoaffective episode should last at least one month, and there should be no relationship between the symptoms of SAD and other diseases and/or the use of psychoactive drugs [15]. In addition, the description of the patient’s clinical condition can be supplemented with the following features: first episode of the disease, multiple episodes of the disease, or continuous course, and the severity (mild, moderate, moderate) of positive, negative, cognitive, manic, depressive, and psychomotor symptoms according to the proposed assessment scale should be indicated [15]. If the patient has no history of schizoaffective episodes and the symptoms persist for at least one month, the diagnosis of “Schizoaffective disorder, first episode” is appropriate. If subclinical symptoms persist for one month or more, including as a result of treatment, a diagnosis of “Schizoaffective disorder, first episode, in partial remission” is made. In a situation where the patient’s current state presents “no clinically significant symptoms” and the condition previously “met the diagnostic criteria for SAD”, the diagnosis of “Schizoaffective disorder, first episode, in full remission” is the right one. If the patient has a history of SAD or schizophrenia episodes and his current state meets the diagnostic criteria for SAD, the diagnosis should be “Schizoaffective disorder, multiple episodes, currently symptomatic”. The qualification of partial and full remission in SAD with multiple episodes is reached in the same way as in SAD with the first episode. If the symptoms of SAD persist for at least one year with possible “short periods of subclinical symptoms”, the diagnosis should be “Schizoaffective disorder, continuous, currently symptomatic”. In this case, the qualification of partial and full remission is also possible [15].
ICD-11 experts have sought to resolve the ambiguities and inconsistencies of ICD-10, particularly the version for clinical use, by stipulating that the patient’s condition must meet the full gamut of diagnostic criteria for schizophrenia — not merely “at least one symptom” — and a moderate-to-severe depressive episode, rather than just “some depressive features” [3]. Additionally, ICD-11 enables one to assess other clinical manifestations (beyond affective symptoms) of SAD using supplementary codes under “Symptomatic manifestations of primary psychotic disorders” [15]. Moreover, the diagnosis of affective disorder with psychotic symptoms may be changed to SAD if psychotic symptoms meet the threshold for schizophrenia.
ICD-11 also outlines key aspects of the differential diagnosis for SAD [15]. In particular, the occurrence of a SAD episode does not preclude a diagnosis of schizophrenia, and vice versa. In both schizophrenia and SAD, at least two characteristic symptoms of schizophrenia must be present during one month or more. However, only in SAD do schizophrenic symptoms coexist with affective symptoms that meet the criteria for an affective episode. On the other hand, in schizophrenia, affective symptoms may occur, but they last less than one month and do not reach the level of the moderate-to-severe depression, manic, or mixed episodes. If an episode initially meets the criteria for SAD, but only affective symptoms recede, and psychotic symptoms without affective symptoms persist longer than their combination does, the case may be diagnosed as an episode of schizophrenia. In a depressive, manic, or a mixed episode of affective disorders, psychotic symptoms may emerge alongside affective symptoms but not meet the diagnostic requirements for schizophrenia (e.g., hallucinations without other schizophrenia symptoms).
According to DSM-5 criteria A, for SAD it is necessary to have concurrently a depressive or manic episode and criteria A for schizophrenia, which includes the presence of at least two of five symptoms (delusions, catatonic behavior, hallucinations, disorganized speech, negative symptoms), wherein the presence of one of the first three is mandatory [17]. That is, in fact, the first criteria for SAD in ICD-11 and DSM-5 coincide, except for the fact that there are no mixed affective episodes in DSM-5. There is also a significant difference between the classifications in the understanding of SAD. Thus, according to the DSM-5 classification, for a diagnosis of SAD it is necessary that the symptoms of mood disorders predominate over psychotic symptoms (criteria C) and that early hallucinations or delusions last for at least two weeks in the absence of depressive, manic, or mixed symptoms (criteria B). The patient description can be further detailed by the classification of SAD as a depressive or bipolar type, the presence of catatonic symptoms, as well as a description of the type of course (first episode, multiple episodes, continuous course) and full or partial remission. In addition, an additional assessment of SAD symptoms is possible according to the Psychosis symptom severity scale (0 to 4 points): hallucinations, delusions, jumbled speech, abnormal psychomotor behavior, negative symptoms, decreased cognition, depression, and mania [17]. Also, DSM-5 indicates that criteria C is intended to differentiate SAD from schizophrenia, and that criteria B is intended to differentiate SAD from depressive or bipolar disorder with psychotic features, in which psychotic symptoms occur only during an affective episode [17].
Table 1 lists the main principles of the SAD diagnosis in different international classifications.
Table 1. Diagnosis of SAD in different international classifications of diseases
|
Classification version (year of publication) |
Title |
Diagnostic features |
Additional features |
|
ICD-9 (1979) |
Header “Schizoaffective type”, chapter “Schizophrenic psychoses” |
Descriptive approach |
Types are not distinguished. Includes: circular schizophrenia, schizoaffective psychosis, periodic schizophrenia. |
|
ICD-10, Clinical Descriptions and Diagnostic Guidelines (1993) |
Header “Schizoaffective disorder”, chapter “Schizophrenia, schizotypal and delusional disorders” |
Categorical approach. Schizophrenic symptoms occur simultaneously or sequentially over several days. At least one symptom of schizophrenia (criteria “A–G”) and at least two symptoms of depression or elation, or mixed bipolar disorders are required. |
Manic, depressive, mixed types are distinguished. The duration and severity of affective episodes are not specified. |
|
ICD-10, Clinical Descriptions and Diagnostic Guidelines (1993) |
Header “Schizoaffective disorder”, chapter “Schizophrenia, schizotypal and delusional disorders” |
Categorical approach. Schizophrenic and affective symptoms co-exist “at least for some time simultaneously” and in relative “equilibrium”. At least one symptom of schizophrenia must be present (criteria “A–D”, “E”, “G”). The duration of depressive and mixed symptoms is at least two weeks, manic – at least one week. Moderate or severe affective symptoms. |
Manic, depressive, mixed types are distinguished. |
|
ICD-11 (2022) |
Header “Schizoaffective disorder”, chapter “Schizophrenia and other primary psychotic disorders” |
Categorical-dimensional approach. Schizophrenic and affective symptoms are present either simultaneously or with an interval of several days. The criteria for schizophrenia and a moderate to severe depressive episode or a manic or mixed affective episode must be met. Episode duration is at least one month. Possibility of qualifying the first and multiple episodes, continuous course, full or partial remission. |
No types distinguished. Additional symptoms may be assessed using additional codes with severity rating (mild, moderate, severe): positive, negative, depressive, manic, cognitive psychomotor symptoms. |
|
DSM-5 (2013) |
Header “Schizoaffective disorder”, chapter” “Schizophrenia spectrum and other psychotic disorders” |
Categorical-dimensional approach. Mandatory presence of criteria A for schizophrenia and criteria for a depressive or manic episode. History of delusions or hallucinations for at least two weeks in the absence of affective symptoms. Predominance of affective symptoms during the episode. Episode duration is at least one month. Possibility of qualifying the first and multiple episodes, continuous course, full or partial remission. |
Bipolar and depressive types are distinguished, as well as a type with symptoms of catatonia. An additional assessment of the state is possible according to the Psychosis Symptom Severity Scale (0 to 4 points): hallucinations, delusions, disorganized speech, abnormal psychomotor behavior, negative symptoms, decreased cognition, depression, mania. |
Conceptualization of schizoaffective disorder
The relationship and boundaries between affective disorders and schizophrenia spectrum disorders remain a central topic of debate in psychiatry [18]. The categorical model suggests that clear distinctions can be made between schizophrenia and affective disorders, leading to the classification of SAD either as a form of schizophrenia, a form of affective disorder, or a distinct condition separate from both. It has been suggested that the classification of SAD as an independent disorder can be viewed as arbitrary and controversial [19]. On the other hand, some patients seem more prone to schizophrenia, while others are more prone to affective disorders [20]. In Russian psychiatry, schizoaffective psychosis has traditionally been regarded both as a favorable variant of shift-like schizophrenia and as a separate disorder. The clinical typology of the episodes was developed with a differentiation between affect-dominant and schizo-dominant forms, based on the duration of symptoms and the degree of progression [21, 22]. As a distinct disease, schizoaffective psychosis is considered a schizophrenic reaction in schizotypal personalities with signs of reactive lability in which schizophrenic psychotic symptoms, although they occur during affective phases, are not pathogenetically related to them [23].
In the review by Potuzak et al. [24], only 7 publications were found that examined how different categories of psychotic disorders differ from the current headers in modern classifications. All the studies have mentioned the need to distinguish one or more classes of psychotic disorders where affective symptoms play an important role. Depending on the prevalence of specific affective syndromes, it was proposed to distinguish the following conditions: SAD, schizomania, schizodepression, and schizobipolar disorder. A subtype with a moderately high level of positive, depressive and manic symptoms and a low level of negative symptoms (bipolar-schizomanic, schizobipolar disorder, affective psychosis, schizoaffective psychosis) was distinguished in five studies; a subtype with a high level of depressive and negative symptoms and a moderate or high level of positive symptoms (schizodepression), in four studies, and a subtype with a high level of manic and positive symptoms and a low level of negative symptoms (schizomania) in two studies. In a sample of 4956 patients with psychotic disorders, seven homogeneous classes of psychoses were identified. The second most common class (after “Kraepelinian schizophrenia”) was the class of “affective psychoses”, accounting for 15% of the patients diagnosed with schizophrenia, characterized by a combination of disorganized thinking, negative symptoms, normal IQ, and a favorable prognosis [25].
The spectrum model assumes that the severity of symptoms is in constant flux, and that individual symptoms fit on a scale, with “pure” affective disorder at one end, “pure” schizophrenia at the other, and SAD positioned in between [17]. It has been shown that diagnostic categories such as schizophrenia, SAD, and BAD do not represent distinct entities but rather reflect areas characterized by certain psychopathological dimensions and neurobiological processes, the boundaries of which are probably arbitrary and are in continuity or overlap with other mental health illnesses, extending even to the edges of “normal” human experience and functioning [26, 27].
An analysis of relevant studies using the dimensional (from “dimension”, degree of severity) model of psychosis showed that the overwhelming majority of studies (31 out of 39) also distinguish an affective dimension, or mania, and depression separately; that is, affective symptoms should be considered not as an additional, but rather as the central component of the psychotic state, along with directly psychotic and negative symptoms [28]. In particular, an analysis of the structure of five groups of symptoms (disorganized thinking, negative symptoms, positive symptoms, depression, mania) in a cohort of 1056 inpatients with psychotic disorders revealed that affective symptoms — rather than negative symptoms or disorganized thinking — are the most distinguishing features of the six identified clusters of psychosis symptoms [27]. A study of associations between three categories of psychotic disorders (schizophrenia, SAD, delusional disorder) and Positive and Negative Syndrome Scale (PANSS) items showed no statistically significant differences between schizophrenia and SAD in terms of negative and positive symptoms, while manic and depressive symptoms were significantly more common in SAD, and there was a continuum of affective disorder severity, with delusional disorder at one pole and SAD at the other [28].
Instead of the concept of a single psychotic spectrum, a model of the so-called metaspectrum was proposed, which includes, in addition to schizophrenia and bipolar, the SAD spectrum [29]. Each spectrum contains various nosologically independent units: from personality traits to clinically complete psychotic syndromes. A three-dimensional model of the spectrum was proposed. The SAD spectrum consists of the following elements (axes):
- SAD in its traditional description, including its various subtypes;
- Leonhard’s cycloid psychosis, confusion psychosis, including motility psychosis, and anxiety-happiness psychosis; other atypical psychoses outside of schizophrenia and affective disorders;
- a subtype of borderline personality disorder with a high proportion of psychotic symptoms in the clinical presentation, which are not sufficient for diagnosing a psychotic disorder.
Clinical and dynamic characteristics of schizoaffective disorder
DSM-5 estimates the lifetime risk of SAD at 0.3%, which is 1/3 higher than in schizophrenia [17]. The incidence of SAD among adult Europeans is 1.1% [6], with “schizomanic” episodes being more common than “schizodepressive” episodes during the first hospitalization; however, depressive episodes are much more common during follow-up than manic ones [30].
Prodromal states in SAD have been more extensively researched in the context of depressive SAD, where a longer prodrome is observed, along with a high prevalence of perceptual disturbances, such as imperative hallucinations, suicidal behavior elements, and paranoid personality traits. A significant frequency of psychotic symptoms is associated with initial diagnoses of non-affective psychotic disorders in the majority (60.6%) of individuals with a final diagnosis of depressive SAD [31]. A follow-up study of individuals at ultra-high risk (UHR) of developing psychosis syndrome, which is characterized by the presence of individual short-term psychotic symptoms, showed that 29.9% of individuals with UHR develop signs of affective disorders within five years: accordingly, UHR can be considered a prodrome of not only psychotic, but also affective disorders [32]. Loss of a parent and divorce in the family are more often associated with an earlier onset of SAD for both sexes. Women with SAD have more often reported a history of sexual abuse in childhood or adulthood; and men, stress associated with work or academic exams [33].
The clinical characteristics of SAD have been studied mainly in comparison with schizophrenia and affective disorders. It has been shown that individuals with SAD experience an earlier disease onset and exhibit higher levels of psychotic symptoms and depression compared with control groups, while many characteristics — clinical, demographic, and psychometric — of SAD map those of schizophrenia more closely than those of affective disorders [34]. The Australian National Survey of Psychosis, which included 1825 patients, found that SAD is characterized by more delusional symptoms and thought disorders, as well as depressive and manic episodes, than schizophrenia [35]. In contrast to bipolar disorder, patients with SAD exhibited more pronounced current positive symptoms, delusions, and thought disorders, as well as a lifetime history of psychotic symptoms, including hallucinations and delusions, but that they experienced fewer manic episodes. Compared with patients with schizophrenia, patients with SAD diagnosed according to DSM-5 criteria show higher rates of suicidality and comorbid anxiety disorders, which is important in terms of differential diagnosis with schizophrenia [1].
The dynamic aspects of SAD have been examined in several studies. A ten-year follow-up of 2524 adolescents aged 14–24 years showed that the presence of psychotic symptoms is associated with a 51% increased risk of developing two or more manic symptoms and a 15% increased risk of experiencing depressive symptoms compared with those who did not present psychotic symptoms [36]. The opposite is also true: the presence of at least three depressive and two manic symptoms increases the likelihood of developing psychotic symptoms by 28% and 37%, respectively. In another study, during a 15-year follow-up of 43,495 individuals with unipolar depression, 2.5% of the cases showed a transition to schizophrenia; and another 1.3%, to SAD, with the diagnosis most often changing during the first years of observation [37]. Earlier long-term follow-up studies had shown that 70% of individuals with SAD may subsequently develop a wide variety of episodes (schizophrenic, schizodepressive, schizomanic, manic, depressive, mixed), and the prognosis is similar to that of affective disorders and is much more favorable than in individuals with schizophrenia [38].
A prospective observation with an average follow-up period of 4.47 years of individuals with the first episode of SAD showed that, 83% of the time, they were in a morbid state, including subsyndromal manifestations. This is significantly higher than the same indicator for individuals with manifest psychotic depression (57.8%) and psychosis in the context of bipolar disorder type I (45%) [39].
A one-year follow-up study of individuals with SAD showed that in 31.6% of cases there was at least one, and in 21.1% of cases — two or more anxiety disorders. The presence of obsessive-compulsive disorder at the beginning of observations was associated with a greater severity of the disease [40], and the comorbid panic disorder with an earlier (by four years) onset of the disease [41]. In addition, complaints of anxiety by patients with SAD may indicate a lower level of global functioning in the future [42]. Comorbid posttraumatic stress disorder and SAD correlate with a worse outcome, a higher number of hospitalizations, and relapses in women [33].
Irritability in the structure of affective episodes in SAD occurs in 27.1% of cases and is associated with a greater severity of mania, depression, suicidality, and a decrease in the quality of life. It persists in more than 1/2 of patients for at least two years, is not associated just with the severity of the disease, and can result in a greater number of symptoms and a more significant decline in social functioning. This suggests it should be considered an independent factor contributing to a less favorable disease prognosis [43].
Cognitive profile of schizoaffective disorder
Neurocognitive and social cognition impairment is common in psychoses; so, neuropsychological assessment is gradually being integrated into the assessment of such patients, which is reflected in the need to assess cognitive functioning in SAD in DSM-5 and ICD-11 [15, 17].
In schizophrenia and SAD, there are common impairments in the neural processing of repeated emotional scenes, measured using the evoked potential method, which is associated with cognitive deficit (emotional processing, response suppression/amplification), rather than with affective symptoms [44, 45].
It has been suggested that insufficient inhibitory behavioral control is associated with symptoms such as impulsivity, aggression, substance abuse, and reckless behavior, which is consistent with our current understanding of the relationship between affective and psychotic disorders through a general deficit in inhibitory control [46].
Patients with SAD have a lower degree of emotion recognition impairment than patients with schizophrenia and a lower overall recognition accuracy of all emotions compared to healthy individuals. Effect sizes indicate a more significant deficit in the recognition of negative emotions associated with threat (fear, anger, disgust) in SAD, which indicates a dysfunction of the limbic structures [47].
A study of neurocognitive parameters, social cognition, as well as the brain structures associated with the processing of social stimuli, using valid test batteries and structural magnetic resonance imaging, showed that schizophrenia and SAD are similar in terms of most of the studied parameters, with the exception of better emotion regulation in patients with SAD [45], which, according to the authors, renders the issue of separating these two disorders in classifications debatable.
Minor differences were found between different subtypes of SAD and schizophrenia. Thus, patients with the depressive type of SAD significantly outperformed the group of patients with schizophrenia in terms of information processing speed, according to the results of the Trail Making Test (TMT-A), which allows one to assess human cognitive abilities, while the group of patients with the bipolar subtype of SAD did not demonstrate significant differences from the schizophrenia group in any cognitive dimension. The obtained data confirm the hypothesis that both types of SAD are heterogeneous and include patients with different cognitive and clinical characteristics [48].
Conversely, a meta-analysis of 31 studies involving individuals with SAD, BAD, and schizophrenia found that patients with the depressive type of SAD exhibit neurocognitive impairments that are closer in severity to those with schizophrenia, while patients with the bipolar type of SAD show less severe impairments than those with schizophrenia, but more pronounced impairments than those with BAD. At the same time, there were no significant differences in the neurocognitive profile of patients with the depressive and bipolar subtypes of SAD. Cognitive impairment increased from BAD to SAD, to schizophrenia. It has been suggested that combining the SAD subtypes could complicate our understanding of the relationship between these three disorders [49].
Comparison of the cognitive status of patients with paranoid schizophrenia and SAD showed that at the remission stage, both disorders are characterized by a decrease in the rate of skill formation and the rate of mental performance and active attention, but compared to patients with schizophrenia, patients with SAD show less pronounced memory and executive function impairment; perseverative additions during immediate recall were also statistically significantly more often observed in SAD [50].
Potential biomarkers of SAD
In patients with SAD and psychotic bipolar disorder, functional magnetic resonance imaging revealed increased randomness of brain signals in the ventromedial prefrontal cortex, while in SAD and schizophrenia, an increased chaotic nature of signals in the dorsomedial prefrontal cortex was noted [51]. Abnormal changes in the areas of the prefrontal cortex are observed only in patients with psychosis, but not in their healthy relatives, which, according to us, allows one to consider this feature as a marker of the disease, rather than a familial trait, and the relevance of the biological approach to the classification of psychoses based on functional neuroimaging data.
Another potential marker is the dynamic functional connectivity of brain regions. Analysis of indicators in psychotic BAD, SAD, and schizophrenia allowed us to establish shared signs of dysfunction compared to healthy controls, including a decrease in the strength of the connection between the thalamus and cerebellum and an increase in the strength binding the postcentral gyrus and the thalamus. On the other hand, only in the case of SAD were differences found between the right, middle, and left inferior frontal gyrus, between the left central sulcus (the sulcus of Rolando) and the left Heschl’s gyrus, between the left cuneus and the right middle temporal area, and between the left gyrus rectus and the left cerebellum [52].
In patients with SAD, structural brain abnormalities are found in various brain regions. Affected gray matter areas include the midline, the inferior and orbitofrontal structures, temporal lobes, left parahippocampal, right gyrus rectus, left fusiform gyrus, and bilateral thalamic nuclei. In the white matter, abnormalities in patients are primarily observed in the corpus callosum and corona radiata. Abnormalities are found predominantly in those brain regions where they have previously been observed in schizophrenia and, to some extent, in bipolar disorder [53]. Another study examined the differences in the shape of the basal ganglia in SAD and schizophrenia [54]. In particular, internal deformation on the anterior ventral surface was observed only in SAD, which may indicate a substrate for affective disorders, but not schizophrenia. Significant anteroventral abnormalities in the putamen observed only in SAD suggest that changes in this region contribute to affective disorders. These findings are consistent with ventral putamen shape changes in individuals with untreated major depressive disorder and BAD and, according to us, may be useful in improving diagnostic accuracy in SAD. Decreased amygdala and hippocampus volumes are characteristic of individuals with schizophrenia, SAD, and bipolar disorder compared with the healthy population. When comparing diagnoses and biotypes in the psychosis spectrum in terms of the volume and shape of the amygdala and hippocampus, a significant decrease in volume compared with healthy controls was found in the left amygdala in SAD, while shape abnormalities in the left and right hippocampus were observed in both SAD and schizophrenia [55].
Finally, the immune system may play an important role in the predisposition, occurrence, and progression of mental disorders. The neutrophil-to-lymphocyte ratio (NLR), monocyte-to-lymphocyte ratio (MLR), and platelet-to-lymphocyte ratio (PLR) are innovative, stable, reproducible routine markers of the systemic immune response [56, 57]. An increase in these parameters was noted in individuals with mood disorders and schizophrenia compared to healthy controls. NLR and MLR were higher in the schizophrenia group compared to SAD and can serve to differentiate these disorders. It has been suggested that in schizophrenia, in contrast to SAD, there is an innate immune response as a cause or consequence of microglial activation [58].
In the available literature, only a few publications are devoted to genetic predisposition to SAD. In particular, a study of individuals from the Ashkenazi population with bipolar I disorder, schizophrenia, and SAD showed that only 6 of 64 candidate genes (DPYSL2, DTNBP1, G30/G72, GRID1, GRM4, and NOS1) are common to schizophrenia and SAD [61]. On the other hand, in patients with bipolar disorder with psychotic disorders and schizophrenia, gene overlap was detected in only two chromosomal regions (13q31 and 22q12) [59].
Perhaps, in the future, when screening large samples of individuals with SAD in international genetic projects, rare copy number variations predisposing to specific psychotic disorders will be uncovered [60]. However, with our current knowledge, it is not possible to separate patients with schizophrenia, BAD, and SAD based solely on genetic research data.
DISCUSSION
Brief interpretation of results
Despite important changes in the ICD-11 and DSM-5 criteria for SAD, revision of these classifications has not resolved many of the issues important for clinical practice, and the concept of SAD remains inadequately defined [61, 62]. During an episode of illness, affective and psychotic symptoms may fluctuate, and, accordingly, the diagnosis of SAD may change. For example, the threshold for SAD may be crossed at some points in time, but not at others. Achieving greater clarity regarding the relationship between affective and psychotic symptoms throughout the illness may require additional information from medical records and from the persons interacting with the patient, and this information cannot always be trusted. In addition, in real life, it may be difficult to determine the onset of an episode of illness, and the period between its manifestation and the seeking of help may be lengthy [63]. Often, it is difficult to identify the moment when affective symptoms play a significant role in the clinical presentation due to massive psychotic symptoms, or medical history information may be unavailable for various reasons, including information regarding a psychotic episode in the past [64]. According to the DSM-5 criteria, a diagnosis of schizophrenia may be erroneously established in the first case and an affective episode with psychotic symptoms in the second case. Also, in the literature on unipolar depression, difficulties in assessing the severity of a depressive episode are noted [65]; therefore, the requirement of both classifications for the presence of moderate to severe depression in SAD may be difficult to meet in practice. An additional source of confusion for clinicians is that both ICD-11 and DSM-5 additionally allow for the description of depressive symptoms in schizophrenia. In DSM-5, the task is simplified by the fact that in order to diagnose SAD, it is necessary to find a history of a psychotic episode without affective symptoms [17]. In ICD-11, only purely affective symptoms (low mood and suicidal thoughts) are included in the classification of depression in schizophrenia, and the assessment of its severity does not coincide with the assessment of the severity of a depressive episode in mood disorders [15].
The views of specialists on SAD often differ from the criteria outlined in classifications. A survey of 113 clinical psychologists showed that, in their opinion, SAD is a “less psychotic” disorder than schizophrenia and “less affective” than bipolar disorder and unipolar depression, which is inconsistent with the understanding of SAD as a disease of lesser severity compared to other psychiatric diseases [30].
Even in international classifications, there is no consensus on the specific subtypes of SAD. The ICD-11 recognizes three subtypes (manic, depressive, and mixed), while the DSM-IV identifies two (depressive and manic), and the DSM-5 distinguishes between bipolar and depressive subtypes. In addition, empirically identified classes of psychoses within the categorical approach also show poor correspondence with the diagnostic categories of DSM-5 and ICD-10. On the other hand, the empirical nature of the identified dimensions of psychoses, including SAD, explain the heterogeneity of clinical symptoms much better than the categories of psychoses [66], and an understanding of psychotic disorder as a “multidimensional syndromic variation with an unpredictable course and outcome” with the introduction of a single concept of “psychotic spectrum” would be generally useful for psychiatry [67], especially given the fact that combination of schizophrenia and affective disorders occurs much more frequently than might be expected on the basis of a random coincidence or common genetic factors [68]. As we criticize the modern concept of SAD for its reductionism and the subjectivity of the preferences of individual clinicians, it is our suggestion that each patient be evaluated holistically, taking into account follow-up data, disease course and pathophysiology, and to identify several discrete forms of these diseases [69].
The study of the cognitive functioning of individuals with SAD is of significant importance for diagnosis and prognosis, and its effective management can reduce the cost of care to such patients both in the short and long term [48, 70]. Neuroimaging changes explain the similarity of clinical manifestations of SAD and BAD with psychotic manifestations on the one hand, and cognitive deficit in schizophrenia and BAD, on the other. It is noteworthy that functional and morphological changes in SAD overlap with both schizophrenia and BAD, the combination of which, together with its unique features and clinical characteristics, allows one to situate SAD in the context of the BAD–SAD–schizophrenia metaspectrum.
Limitations
The possible limitations of our work are related to the lack of a unified concept of SAD, the small number of studies identifying SAD (and particularly its subtypes) in distinct groups or subgroups, and the small sample sizes of patients with SAD included in the studies. In some studies, the differences between the groups resided at a subclinical level.
CONCLUSION
Despite important changes in the diagnosis of SAD in ICD-11 and DSM-5, many unresolved issues persist as regards this disorder from the point of view of clinical psychiatry and neurobiology. There has been some improvement in the inter-rater reliability of SAD criteria in modern classifications, but this has not yet led to a clearer understanding among specialists, while the various subtypes of SAD identified so far fail to account for the heterogeneity in the clinical presentation. Apparently, the dimensional approach to the conceptualization of SAD, according to which the intensity of psychotic and affective symptoms can fluctuate over time and they can influence one another, more accurately reflects the disease's variability. Basic research also does not support the identification of a distinct cognitive, neuroimaging, or immunological SAD endophenotype that differs qualitatively from schizophrenia and affective psychoses, which, according to some authors, justifies the use of the SAD category, despite the clinical uncertainty surrounding this diagnostic header. The conceptualization of SAD remains incomplete, and new approaches rooted in neuroscience appear to be needed to better understand the coexistence of affective and psychotic symptoms.