INTRODUCTION
Schizophrenia is a severe mental disorder, the burden of which is growing worldwide [1]. The prescription of antipsychotic therapy is the primary medical intervention for this disorder [2]. Antipsychotics are traditionally divided into first- and second-generation ones. A unifying feature of first-generation antipsychotics is direct blockage of D2 dopamine receptors [3]. The discovery of clozapine and attempts to replicate its unique receptor profile led to the second generation of antipsychotics [4]. Second-generation antipsychotics have a high affinity for other receptors, such as serotonin 5-HT2A, histamine H1, and others [4–6]. A subgroup of antipsychotics, the main feature of which is partial agonism to D2/D3 receptors (third generation), has been recently separated from second-generation ones [3, 4, 6]. At the time of writing, aripiprazole, brexpiprazole, and cariprazine are recognized to have this property.
The choice of a particular antipsychotic for treatment is a multidimensional undertaking, because not only the manifestations of the disease and comorbidities have to be considered, but the pharmacological profile of the drugs as well [7]. There is a large number of drugs, each of which is characterized by its profile of therapeutic activity and safety [5, 8]. An unambiguous recommendation of a specific name, clozapine, is justified only in the case of resistant forms of schizophrenia [9]. There is also no consensus in the current clinical guidelines on the choice of the generation of antipsychotics [5, 10]. In summary, there are no hard-and-fast rules when it comes to choosing a particular drug for the treatment of schizophrenia [11].
Yet, in practice, physicians regularly make the decision to prescribe a specific drug. That choice is thought to be driven by two main criteria: the probability that the drug will be effective in treating the disease (efficacy), and the probability that side effects will not occur as a result of the use of the drug (safety) [12–14]. Two factors have a direct impact on the decision-making process in the clinical context: “factor one”, which is intuitive, automatic, based on experience and affect, and “factor two”, which is analytical, slow, verbal, and logical [15, 16]. To date, the important role of unconscious factors (“factor one”) in clinical decision-making has been demonstrated [16]. For instance, the “belief” in the efficacy and safety of second-generation antipsychotics is a stronger argument in favor of prescribing a drug than the data rebutting it [13]. The choice of which generation of antipsychotics to use is influenced by a physician’s practice [17, 18], which indirectly confirms the role of experience in the decision-making process. Thus, in addition to efficacy and safety, medical decision-making is influenced by a variety of factors, such as cognitive errors [19].
Despite the large body of existing research, there is still a need for further study into the decision-making process. We could not find any research that assesses the quantitative differences in drug perception, and this has been the reason behind our decision to undertake the present study. We have anticipated that the perceptions of psychiatrists would not align with the reference differences between drugs known from meta-analyses. Preference would, as a matter of course, be given to a drug with a long history of use; i.e., risperidone. To test this hypothesis, statistical theories were formulated: (a) the structure of the responses fully aligns with the structure of initial differences between drugs, and (b) the parameters of a quantitative assessment of perception and the decision-making process match the initial data. The rejection of these statistical hypotheses would be confirmation of our research hypotheses.
METHODS
Target selection
The object of the study is the subjective evaluations of psychiatrists working in the public health care system regarding the choice of antipsychotics. The questionnaires were distributed to psychiatrists of the state system. The requirements for completing the questionnaire are as follows:
- A valid credential that confers the right to provide medical care in the field of psychiatry;
- Experience in using cariprazine for the treatment of schizophrenia — more than 5 treated patients;
- Experience in using risperidone for the treatment of schizophrenia — more than 5 treated patients.
The return of a completed questionnaire has been viewed as confirmation of consent to participate in the study.
Sample
A quota sampling strategy was used to recruit participants to the study. In each state health care center (3 psychiatric hospitals, 2 psychiatric hospitals with an out-patient unit, 10 psychoneurological out-patient clinics), psychiatrists were invited to fill out a blind questionnaire. The place of practice (outpatient clinic, day hospital, 24-hour hospital) was a quota characteristic. An equal number of two versions of the questionnaire was assumed for each quota.
Since the survey did not require any information from the participants, the study did not need an ethics review. The participants were assured that every attempt would be made to ensure that their responses to the questionnaire remained confidential. Administrative coercion was excluded in the sampling process. The return of an anonymized questionnaire was considered to be indicative of informed consent. These considerations are in keeping with the ethical principles set out in the Helsinki Declaration.
Drug comparison model
Where an object’s properties can be described using an interval value system, the difference on the measurement scale provides a quantitative measure of the difference. This approach is inappropriate for cases where measurement tools are unavailable or reliant on a value judgment system. Violation of the equidistant principle can result in inaccurate assessment of classical measures such as total score and arithmetic mean and hinder the use of statistical models like linear regression and analysis of variance [20]. For this category of data, ordinal regression is the appropriate approach [21]. The detailed rating model is presented in the Appendix A (in the Supplementary).
The described methodology utilizes classical sensometric protocol of 2-Alternative Forced Choice, with the option of “No difference” (2-AC protocol) [22]. This protocol is commonly employed to determine product preference. In this particular study, we sought to evaluate psychiatrists’ perception of a drug’s specific attribute severity, based on their individual professional experience.
Variables
The drugs risperidone and cariprazine have been chosen for comparison. Risperidone is the oldest second-generation antipsychotic [4]. At one point, experts regarded it as the drug of choice for the treatment of schizophrenia [23]. Risperidone is the most commonly prescribed medication [13], and this is also true in Saint Petersburg, as shown in the current study [24]. In addition to the recognition and wide popularity of risperidone, that choice has influenced its use as a comparison drug in studies of cariprazine [25–27].
Cariprazine is a new antipsychotic being a partial agonist of D3/D2 dopamine receptors that predominantly effects D3 receptors. The drug was created based on several assumptions: affinity for the D2 receptor is mandatory, and that partial agonism or antagonism to D3 receptors can improve cognitive functions and reduce the risk of catalepsy. In addition, the drug is believed to have a greater affinity for D3 receptors [28]. Cariprazine is effective against the core symptoms of schizophrenia, including the first psychotic episode, and it has good tolerability [29–31]. There is evidence that Cariprazine is highly effective in patients who display predominantly negative symptoms [25–27, 32].
The meta-analyses by Pillinger et al. [33] and Huhn et al. [8] were used to create a drug comparison model. All network analyses served as a basis: overall change in symptoms, all-cause discontinuation, positive symptoms, negative symptoms, depressive symptoms, weight gain, use of antiparkinsonian drugs, akathisia, increased prolactin levels, QT interval prolongation, sedation, anticholinergic side effects [8], increase in total cholesterol, low-density (LDL) and high-density (HDL) lipoproteins, triglycerides, and glucose [33]. The use of the surface under the cumulative ranking curve allowed us to identify three superiority positions for risperidone, ten comparable positions, and four cariprazine superiority positions (3 – 10 – 4). This distribution of the results is described by the theoretical parameters δ 0.147 and τ 1.167. For identifying the calculated figures, 928 responses are required (power — 80%, confidence probability — 95%).
To simplify our study, we analysed the positions under consideration to determine if any could be excluded. The first excluded characteristic was a general change in symptoms. Usually, the total score is calculated as a sum of subscales. As there are distinct sources of data on the negative and positive symptoms, it becomes unnecessary to incorporate the overall score into the analysis. The exclusion of all-cause discontinuation is related to the inability to conduct an evaluation in clinical practice. The need for antiparkinsonian therapy does not fully reflect the assessment of the occurrence of parkinsonism; so, it has been excluded. Exclusion of two positive characteristics for risperidone and one neutral characteristic resulted in an excessive optimistic difference between the drugs (1 – 9 – 4, δ — 0.636, τ — 1.436, 54 observations).
Furthermore HDL, LDL, and triglycerides have not been included in the experiment for the following reasons: total cholesterol is a composite of lipoproteins and provides the best standardization in laboratory testing compared to other lipids and lipoproteins [34]. Since total cholesterol is a composite of lipoproteins, replacing the indicator to simplify the model is warranted. Replacing several parameters with one common variable is a means to simplify the model. In particular, the exclusion of the laboratory measure of LDL, for which cariprazine is shown to be beneficial, is a way to partially balance out the exclusion of the two positive characteristics of risperidone. Thus, for identifying the difference between the answers in the sequence “Risperidone” — “No difference” — “Cariprazine” (1 – 7 – 3), 79 questionnaires have been required (δ — 0.517, τ — 1.372).
In addition to the quantitative differences between the drugs (parameters δ and τ), qualitative differences in perception can captured. Quantitative differences do not reflect the conformity of the responses to the initial therapeutic profile of the drugs. Therefore, the qualitative dimension of the perception is assessed by the proportion of the respondents’ answers matching the original perceived advantages. Table 1 presents the number of included parameters and the decisions made for each drug’s property in the meta-analyses.
Table 1. The cariprazine and risperidone characteristics used in the study
|
Parameter |
Average value [95% CI] |
Advantage per meta-analysis |
|
“+” Positive symptoms (SMD) |
-0.30 [-0.46; -0.15] |
Risperidone |
|
“+” Negative symptoms (SMD) |
-0.04 [-0.17; 0.08] |
No difference |
|
“+” Depressive symptoms (SMD) |
0.14 [-0.15; 0.43] |
No difference |
|
“−” Weight gain (kg) |
0.71 [-0.09; 1.51] |
No difference |
|
“−” Akathisia (RR) |
0.79 [0.50; 1.37] |
No difference |
|
“−” Prolactin elevation (ng/ml) |
41.17 [34.63; 47.74] |
Cariprazine |
|
“−” QT interval prolongation (ms) |
6.22 [1.58; 11.01] |
Cariprazine |
|
“−” Sedation (RR) |
1.79 [1.14; 3.23] |
Cariprazine |
|
“−” Anticholinergic effects (RR) |
0.91 [0.56; 1.54] |
No difference |
|
“−” Cholesterol (SMD) |
0.15 [-0.02; 0.31] |
No difference |
|
“−” Glucose (SMD) |
-0.18 [-0.50; 0.14] |
No difference |
Note: SMD (Standardized Mean Difference), RR (Relative Risk), and CI (Confidence Interval), “+” is used to indicate that the advantage has been evaluated according to the greater relative severity of the effect, while “−” means the advantage is determined by the smaller relative magnitude of the impact.
The introductory part of the questionnaire included questions regarding the length of service, place of work (outpatient service, day hospital, 24-hour inpatient hospital), prescription of the drugs, and the number of treated patients. The first question of the main part concerned the immediate choice between cariprazine and risperidone in as uncertain circumstances as possible. The second question asked the respondent to compare in pairs the three factors that are most important in prescribing the drugs: the accessibility and availability of discounted drug coverage, the efficacy of the drug, and the side effect profile. Since these two questions are the subject of a separate analysis, they are not used in this paper.
The third portion of the questionnaire included questions related to the comparison of the drugs based on selected variables from the meta-analysis. The questions began with: “According to your experience and clinical practice, choose the drug that in your opinion…”. The third portion is divided into three subgroups of parameters reflecting the therapeutic effect, side effects, and changes in objective parameters. Since some of the questions reflect the worst characteristic (for example, weight gain), reverse order for calculations is used, but the evaluated categories remain the same. To counteract the effects of consistency, all questionnaires are divided into two versions. In questions 1 and 3 of the first version, the list of answers began with cariprazine; and in the second — with risperidone. In question 2, the sequence of comparison pairs is mirrored in each of the versions. This approach meets the requirements of the 2-AC protocol [35]. Appendix B (in the Supplementary) provides an example of the questionnaire that is offered for completion.
Statistical analysis
Absolute values and prevalence, n (%), were used to describe categorical variables. Variables with a continuous distribution were described by a mean (M). When necessary, 95% confidence intervals for the calculated parameters (lwr; upr) were provided. The minimum and maximum values (|min;max|) were also calculated.
The planned number of respondents was calculated using the twoACpwr function, and the discrimination parameters were calculated using the twoAC function1. For repeated measures, the number of responses required was equated to the number of questionnaires required. The description of the 2-AC protocol and its technical implementation are provided by the developer of the sensR library [22]. Alignment of the response pattern with the results of the meta-analysis has been verified using the opa library2, which was designed to make sure that the observed response structure corresponds to a hypothetical distribution [36]. The percentage of correct classifications (PCC) and the coefficient of randomness of the result (c-value) were calculated. To determine the relative difference in the responses [37], calculation of a multinomial distribution with a 95% confidence interval using the function MultinomCI was employed3. The relationship between variables was estimated by ordinal regression4. The optimal model was chosen according to the lowest Akaike’s criteria (AIC). The strength of association between variables was represented as an odds ratio and a 95% confidence interval (OR [lwr; upr]). All calculations were performed in the Rv4.2.3 programming language5.
RESULTS
A total of 79 psychiatrists were interviewed anonymously. The psychiatrists had an average experience of 11.0 (9.4, 12.7) years in the specialty, with a minimum of 2 years; and a maximum of 40 years. Cariprazine was used for a period of 5 to 48 months, whereas risperidone was used for 11 to 264 months. The number of patients treated with cariprazine was subjectively assessed to be 10.5 (8.6, 12.5), while the same figure for risperidone was 360.1 (95.3, 624.9). The distribution of physicians according to their practice setting was as follows: outpatient clinic — 34.1%; day hospital — 32.9%; and 24-hour hospital — 32.9%.
Table 2 displays the distribution of responses regarding the clinical difference between the drugs. When analyzing whether the pattern of respondents’ answers matched the hypothesis behind the model, only 44.37% (c <0.001) matched the hypothesis.
Table 2. Distribution of answers on the perception of the clinical difference between drugs
|
Parameter |
Risperidone (proportion, [95% CI]) |
No difference (proportion, [95% CI]) |
Cariprazine (proportion, [95% CI]) |
Advantage by meta-analysis |
Advantage by survey results |
|
“+” Positive symptoms |
0.57 (0.43; 0.72), n=45 |
0.42 (0.28; 0.57), n=33 |
0.01 (0.00; 0.17), n=1 |
Risperidone |
Risperidone |
|
“+” Negative symptoms |
0.01 (0.00; 0.06), n=1 |
0.05 (0.00; 0.10), n=4 |
0.94 (0.89; 0.99), n=74 |
No difference |
Cariprazine |
|
“+” Depressive symptoms |
0.04 (0.00; 0.16), n=3 |
0.24 (0.13; 0.36), n=19 |
0.72 (0.61; 0.84), n=57 |
No difference |
Cariprazine |
|
“−” Akathisia |
0.32 (0.16; 0.48), n=25 |
0.42 (0.27; 0.58), n=33 |
0.27 (0.11; 0.43), n=21 |
No difference |
No difference |
|
“−” Anticholinergic symptoms |
0.03 (0.00; 0.16), n=2 |
0.70 (0.58; 0.83), n=55 |
0.28 (0.16; 0.41), n=22 |
No difference |
No difference |
|
“−” Sedation |
0.00 (0.00; 0.11), n=0 |
0.23 (0.13; 0.34), n=18 |
0.77 (0.67; 0.88), n=61 |
Cariprazine |
Cariprazine |
|
“−” Weight gain |
0.01 (0.00; 0.11), n=1 |
0.16 (0.08; 0.26), n=13 |
0.82 (0.73; 0.92), n=65 |
No difference |
Cariprazine |
|
“−” QT extension |
0.00 (0.00; 0.09), n=0 |
0.86 (0.78; 0.95), n=68 |
0.14 (0.06; 0.23), n=11 |
Cariprazine |
No difference |
|
“−” Increase in prolactin |
0.00 (0.00; 0.11), n=0 |
0.23 (0.13; 0.34), n=18 |
0.77 (0.67; 0.88), n=61 |
Cariprazine |
Cariprazine |
|
“−” Increase in glucose |
0.01 (0.00; 0.13), n=1 |
0.76 (0.66; 0.88), n=60 |
0.23 (0.13; 0.35), n=18 |
No difference |
No difference |
|
“−” Increase in cholesterol |
0.00 (0.00; 0.10), n=0 |
0.82 (0.73; 0.92), n=65 |
0.18 (0.09; 0.28), n=14 |
No difference |
No difference |
Note: n — number of observations, CI — confidence interval. “+” — the advantage was evaluated according to the greater relative severity of the effect, “−” — the advantage was evaluated according to the lesser relative severity of the effect.
Upon analysis of the response profile, it was found that most respondents viewed risperidone (0.57 [0.43; 0.72]) as superior in terms of efficacy towards positive symptoms. However, this did not differ from the proportion of those who reported a comparable antipsychotic effect between the drugs (0.42 [0.28; 0.57]). The advantage of cariprazine was overwhelmingly noted regarding its impact on negative (0.94 [0.89; 0.99]) and depressive (0.72 [0.61; 0.84]) symptoms. Cariprazine was also rated as safer than risperidone as relates to the risk of the following side effects: sedation (0.77 [0.67; 0.88]), weight gain (0.82 [0.73; 0.92]), and increase in prolactin (0.77 [0.67; 0.88]). The risk of anticholinergic side effects (0.70 [0.58; 0.83]), QT interval prolongation (0.86 [0.78; 0.95]), increased glucose (0.76 [0.66; 0.88]), and cholesterolemia (0.82 [0.73; 0.92]) was rated as comparable between the drugs. Most respondents rated the risk of akathisia as comparable (0.42 [0.27; 0.58]), but this was not unlike the proportion of those who considered cariprazine safer (0.27 [0.11; 0.43]).
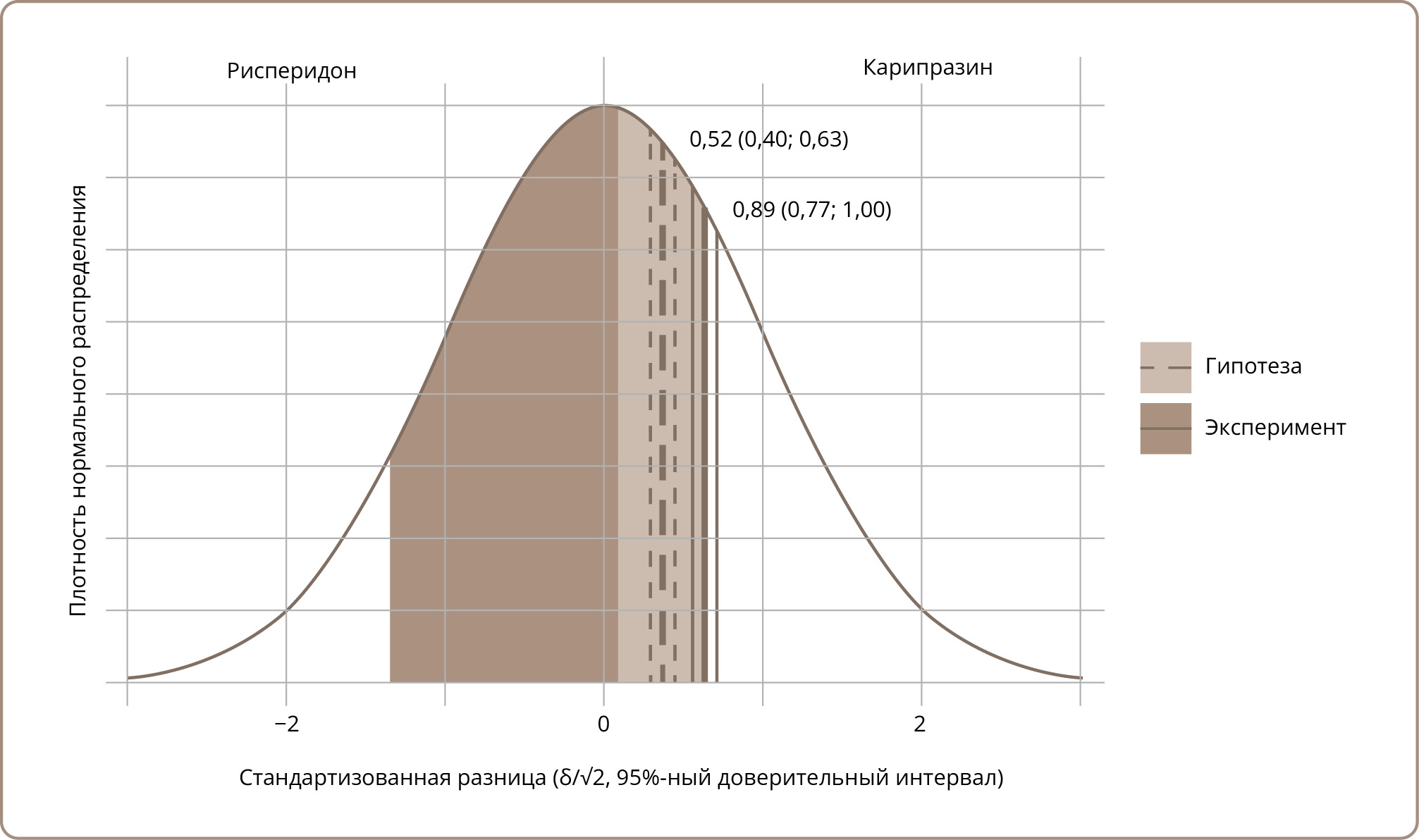
When calculating the parameters of perception, the results showed that δ was 0.889 (0.774, 1.004) and τ was 1.001. The discrimination index obtained was higher than the calculated one, their confidence intervals not overlapping (the prior value being 0.517 [0.404, 0.630]). The boundary of the “No difference” category was lower than the calculated one (prior τ being 1.372). The results suggest a statistically significant difference between the hypothesis and empirical data.
Figure 1 illustrates the difference between hypothetical and empirical parameters.
Figure 1. Standardized distribution of the differences between the drugs
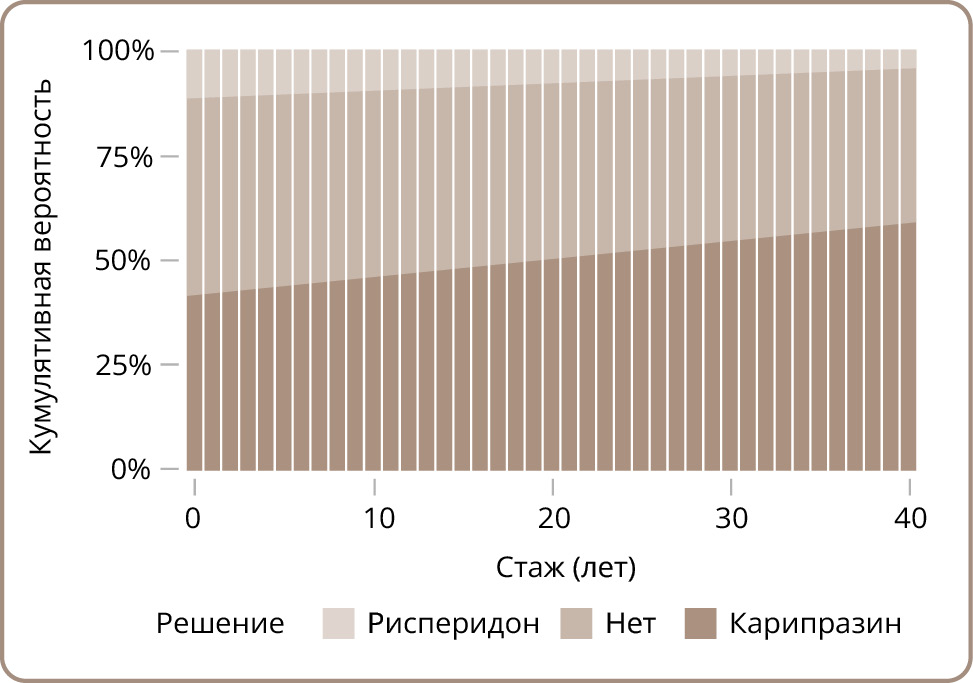
In addition, the hypothesis of the impact of the length of service on the perception of the differences between the drugs was tested. Adding age as a covariate improved the model’s performance (AIC 1622.97 vs. 1624.94). The length of service parameter exceeded the classical threshold of statistical significance (0.01 [0.00; 0.02], p=0.047). There was also a correction of the δ parameter towards a slight decrease (0.725). Figure 2 shows that the probability of choosing cariprazine increases with an increase in the length of experience, while the proportion of responses “No difference” and “Risperidone” drops. There was no correlation identified between the correspondence of the hypothetical pattern responses from individual participants and their length of work experience (p=0.870).
Figure 2. Probability of response depending on the length of service as a psychiatrist
DISCUSSION
The aim of this study was to assess the quantitative differences in psychiatrists’ perceptions of the efficacy and safety of various antipsychotics. The 2-AC protocol was used to quantify the difference in perceptions and compare it with a reference difference. The meta-analyses were used as a reference, and the reference values of the discrimination and decision-making parameters were calculated: δ — 0.517, τ — 1.372. Based on questionnaire data, the parameters had the following values: δ — 0.889, τ — 1.001. The qualitative aspect of the perception was also evaluated. The complete agreement of the response structure with the data from the meta-analyses is equivalent to 100% PCC. The observed value of 44.37% indicates a disagreement between the perception structure of psychiatrists and the objective data. Thus, the first part of the hypothesis of the study was confirmed. Regarding the perception bias in favor of risperidone, the hypothesis was not confirmed.
Every day physicians make decisions, the consequences of which affect the lives of patients and society as a whole [38]. Even in the face of considerable uncertainty, the physician is in a position to anticipate consequences and come up with solutions. The cost of this ability is cognitive distortions and errors that can negatively affect the final result [19]. For this reason, it is necessary to implement decision-support systems, the task of which is to minimize the number of erroneous decisions [12]. How such a system functions depends on the input information and algorithms provided by the developers. For example, it is easy to implement a drug selection protocol for the treatment of delirium with an extrapolation of the QT interval [39]. It is much harder to objectify the rating of antipsychotics [40].
The creation of the Personal Antipsychotic Choice Index [40] has been one attempt to generate such a ranking system based on therapeutic activity profiles in drug trials by expert evaluation. Direct ranking based on numerical characteristics has been performed only in large meta-analyses [8, 33]. However, these studies do not take into account the perception of therapeutic efficacy from both physicians and patients. Patients have been known to prefer drugs that result in less fatigue and memory problems [41]. The studies also uncovered a difference in the way doctors and patients choose drugs [42], which we believe is related to the perception of the disease itself and one’s idea about how to manage it.
The perception of the therapeutic properties of drugs is a specific case of this general problem. Both assumptions and decisions depend on background information. This is illustrated in this case study. The authors of the meta-analysis [8] intentionally have excluded studies on the therapeutic effects on patients with predominant or prominent negative symptoms, since that issue is the subject of a separate study [43]. For this reason, the initial hypothesis assumed no difference between risperidone and cariprazine in terms of effectiveness as relates to negative symptoms. However, a significant difference in the perception of clinicians is obvious as concerns cariprazine and risperidone with respect to their effect on negative symptomatology, since there was no specific subgrouping of patients in practice.
For depressive symptoms, the situation is probably similar. The expected difference between cariprazine and risperidone in terms of their effect on depressive symptoms is insignificant (0.14 [-0.15; 0.43]) [8], although in practice this effect is perceived as strong. The result can be explained by the fact that the overall score is the result of a number of factors. For example, it is difficult to distinguish between negative symptoms and symptoms of depression [44, 45]; so, it cannot be excluded that the antidepressant effect reflected in the physicians’ experience might be due to a change in the severity of the negative symptoms. It should be noted that the original data relate only to depressive symptoms within a psychotic episode, whereas depression in schizophrenia is a more complex condition [46].
This may also explain the difference in QT interval estimates and weight gain rather than actual clinical effects. A QT interval shortening of -1.45 (-6.20; 3.20) ms is known to occur regarding cariprazine in comparison with placebo, whereas an increase in the interval by 4.77 (2.68; 6.87) ms has been proven for risperidone [8]. However, these results indicate a statistical difference that may not coincide with the practical significance [47]. In our opinion, if physicians had not noticed the critical complications that accompanied the drugs, they might not have noticed any difference between cariprazine and risperidone (despite the fact that cariprazine is safer as relates to this parameter). Strangely enough, a similar structure of differences regarding weight gain has been perceived differently. On cariprazine, the average increase in body weight is less than one kilogram (0.73 [-0.06; 1.52]), which is comparable to the placebo. Risperidone, as has been shown, can increase body weight by more than a kilogram (1.44 [1.05; 1.83]). It would seem that the conclusion should be identical as when assessing cardiac activity, but weight gain worries patients [41, 42] and they are more likely to insist on this problem in their complaints. On the other hand, physicians are also concerned about the risk of weight gain in patients [42]. This may explain why the statistically insignificant difference between cariprazine and risperidone (0.71 [-0.09; 1.51], kg) is a perception of the superiority of cariprazine.
Finally, an explanation is needed regarding the effect of the length of experience of the physicians on the discrimination index. We anticipate that a lengthier service has to increase the number of “Risperidone” responses. This conviction is based on the preference for first-generation antipsychotics by physicians with more experience [17, 18]. In a similarly way, an “older” drug like risperidone would have been perceived as preferable, but that assumption has not been confirmed. In our opinion, the evaluation of efficacy and safety does not align with the decision about the choice of a drug. Further research is needed to understand how and why the length of experience affects the perception of the differences between drugs.
Limitations
The first limitation relates to the subjective choice of the initial parameters in creating the model. The model was based on the results of two network meta-analyses and does not include all the therapeutic properties of the drugs. In addition, when simplifying the study model, it proves impossible to evenly exclude the advantages of the drugs. Therefore, the set of tested variables cannot be upheld as perfectly balanced. The second limitation has to do with location. All the physicians in the study have practiced in public institutions in Saint Petersburg, Russia. We believe that in other cities and regions of Russia, the results might be different. Thirdly, the results cannot be considered as a guide to a particular set of actions in clinical practice. Fourth, it would be misguided to judge the therapeutic properties of the drugs from these results, as the aim of the study was to assess the perceptions of mental health practitioners, not to evaluate the drugs as used in clinical practice.
CONCLUSION
To the best of our knowledge, the current study is the first to parameterize safety and efficacy characteristics using the sensometric theory. For the first time, a quantitative difference in the perception of the therapeutic properties of antipsychotics has been uncovered using cariprazine and risperidone as examples. Clinicians routinely perceive differences between drugs, and these differences are starker than expected. The pattern of perceived differences is not fully consistent with the results of clinical trials. This result can be considered when updating clinical guidelines and further developing decision-support systems.
Authors’ contribution: Alla Dobrovolskaya, Aleksandr Sofronov: study design development, final editing of the manuscript text; Galina Prokopovich: questionnaire development, obtaining data for analysis; Anton Gvozdetckii: data analysis, manuscript writing. All authors contributed substantially to the study and the article, and read and approved the final version before publication.
1Christensen R, Brockhoff P (2023). sensR: Thurstonian Models for Sensory Discrimination. R package version 1.5-3. Available online: https://CRAN.R-project.org/package=sensR
2Beechey T (2023). Opa: An implementation of ordinal pattern analysis. Available online: https://CRAN.R-project.org/package=opa.
3Signorell A (2023). DescTools: Tools for Descriptive Statistics. R package version 0.99.48.; 2023. Available online: https://CRAN.R-project.org/package=DescTools
4Christensen RHB (2023). Ordinal - Regression Models for Ordinal Data. R package version 2022.11-16.; 2022. Available online: https://CRAN.R-project.org/package=ordinal
5R Core Team (2023). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: https://www.R-project.org/