INTRODUCTION
Autism is a congenital disorder of the central nervous system formation, with variable etiology and pathogenesis, that manifests itself in social and communication skill impairment and repetitive, stereotypical behavior [1]. In most cases, the causes of autism are closely tied to the genes that affect the maturation of synaptic connections in the brain, but the genetic mechanisms of this disease are quite complex [2–4]. Due to the diversity of causes and mechanisms, there is no single established drug therapy. Pharmacotherapy is confined to affecting individual manifestations (aggression, agitation, anxiety) and not the key disorders (communication, social skills, sensory impairment, intelligence, and speech) [5–6]. The effectiveness of the applied methods is quite low, and in most cases patients with autism are unable to work [7].
Due to the lack of effectiveness of standard therapy, the development of new treatments for autism is becoming a priority. One of the most important and promising areas in neurology and psychiatry is the use of cell technologies; namely, methods of treatment using stem cells [8, 9]. One of the most accessible sources of stem cells is cord blood. There have been studies of allogeneic nucleated cord blood cells (CBCs) used in cerebral palsy [10, 11], stroke [12], perinatal encephalopathy [13], Alzheimer’s disease [14], schizophrenia [15, 16]. Using nucleated CBCs in patients with autism is of even greater interest [17–19].
This article presents the results of therapy with nucleated CBCs in a patient with autism and intellectual disability. The use of CBCs was prompted by the ineffectiveness of standard therapies on this patient.
CASE REPORT
An 8-year-old male patient came to a psychiatrist with his parents. His mother presented complaints about the child’s developmental delay, hyperactivity, episodes of excitement with aggression, motor stereotypies, as well as significant speech and communication disorders. The purpose of the visit was to select therapy and define the contours of the rehabilitation measures.
Medical history
The child had developed normally during the first two years. At the age of two years, after a severe ARVI with hyperthermia of up to 40 degrees, changes in his behavior appeared in the form of aggression and motor disinhibition; the child lost his previously acquired speech skills, stopped responding to treatment, and started manifesting stereotypical behavior.
The parents turned to a psychiatrist for the first time when the child was three years old; he was diagnosed with atypical autism with mental retardation (F 84.11) in accordance with the ICD-10 criteria. From the time of the diagnosis, the patient has regularly received antipsychotics and antidepressants to treat behavioral disorders, and by the time of the visit he was taking chlorprothixene at a dose of 45 mg/day. Drug therapy produced no significant improvement; significant behavioral disorders persisted, manifesting themselves in the form of aggressive and auto-aggressive reactions, impulsivity, emotional instability, and severe cognitive and speech disorders. At the age of 8, the child underwent magnetic resonance imaging (MRI) of the brain and electroencephalography (EEG). MRI showed no significant changes; electroencephalography indicated the absence of epileptiform activity.
Prior to the start of therapy with CBCs, the child’s speech development corresponded to level 1 general speech underdevelopment with a pronounced sensory component and the level of intellectual development corresponded to moderate intellectual disability.
At the time of the patient’s request for medical care, the Department of Pediatric Psychiatry at the V.M. Bekhterev National Medical Research Centre for Psychiatry and Neurology was conducting a research study titled “Use of nucleated cord blood cell concentrate in children with severe cognitive deficit with a decreased contact level or autistic manifestations” (the study protocol was approved by the LEC of the V.M. Bekhterev NMRC PN on June 22, 2017, and approved at the meeting of the Academic Council on June 28, 2017; the study was conducted from September 2017 to June 2021). Given the low efficacy of standard therapy and the patient’s eligibility, his parents were offered participation in this study. After a positive decision, the parents signed a voluntary informed consent to participate in this study, as well as an informed consent to the publication of the case report.
PRODUCT CHARACTERISTICS AND ADMINISTRATION PROCEDURE
CBC samples were taken after cutting the umbilical cord, according to the generally accepted method as part of a gratuitous donation on the basis of a voluntary informed consent signed by pregnant women hospitalized at National Medical Research Center For Obstetrics, Gynecology And Perinatology Named After Academician V.I. Kulakov. Within a maximum of 4 hours after sampling, the samples were delivered to the laboratory, where the CBCs were isolated according to the preparation procedure used in oncological hematology as an alternative to donor bone marrow [20]. The CBC concentrate was poured into cryotubes and stored in liquid nitrogen at -196°C. Blood samples from the mother and cord blood samples were tested for sterility and blood-borne infections by an independent laboratory. The cord blood samples were tested and found negative for human immunodeficiency virus (HIV-1/2, antigen/antibody), hepatitis B (HBs Ag, anti-HBc-total) and C (anti-HCV-total), T-cellular leukemia (anti-HTLV-1/2), herpes simplex viruses type 1 and 2 (anti-HSV IgM), cytomegalovirus (anti-CMV IgM), pathogens of toxoplasmosis (anti-Toxo IgM), syphilis (Syphilis RPR), bacteria, and fungi. The samples were simultaneously characterized in terms of AB0/Rh and the content of CD34 cells [21, 22].
Before clinical use, the CBCs were thawed under aseptic conditions, washed from the cryoprotectant, assessed for viability using the trypan blue test, and placed in an infusion medium. The finished product is a sealed polymer container with 20 ml of a pale pink opalescent liquid containing a suspension of 250±50 million nucleated CBCs in a sterile saline solution, with the addition of rheopolyglucin and human serum albumin. The cells thawed and washed from the cryoprotectant were transported to the department in a thermal container at +1...+4°C (on ice). The time from the moment the concentrate was thawed to the start of administration to the patient did not exceed 2 hours. The patient was administered an intravenous injection of cord blood cell concentrate compatible in terms of blood type and Rh factor at a dose of 250±20 million cells per injection. There was a total of 4 injections with an interval of 14 days. In accordance with the study protocol, the patient had his drug therapy completely discontinued before the start of CBC administration (at the time of admission, the child was receiving chlorprothixene at a dose of 45 mg/day).
METHODS FOR ASSESSING CHANGES IN THE PATIENT’S CONDITION
To assess cognitive functions, separate subtests of the Wechsler method were used before the start of therapy and after 6 and 12 months. To assess the changes in autistic manifestations, the Checklist for autism spectrum disorders (CASD) and Autism treatment evaluation checklist (ATEC) questionnaires were completed before the start of therapy and after 6 months. CASD records the presence or absence of 30 characteristic symptoms to differentiate autism from other developmental disorders. This method detects autism in children with 99.5% accuracy and is intended for children aged 1 to 16 years [23]. ATEC is a method for evaluating the effectiveness of ongoing treatment in autism. The test questions are divided into 4 groups: speech and communication, socialization, sensory and cognitive abilities, and health and behavior [24].
Among the subtests of the Wechsler scale, the following ones were chosen: “Digit span”, “Picture completion”, “Kohs blocks”, and “Coding”. The “Digit span” subtest assessed working memory and active attention; the “Picture completion” subtest was used to assess perceptual abilities, observation, and concentration; the “Kohs blocks” subtest was used to assess analytical and synthetic abilities; while the “Coding” subtest helped assess attention characteristics and development of hand-eye coordination [25].
The instrumental method involves studying brainstem auditory-evoked potentials (BAEPs) of the brain before the start of treatment and after 6 months. The essence of this method is registration of the electrical responses of the brain to auditory stimuli. The method was used to detect abnormal connection between the ears and the brain and helped to assess the functional state of the structures of the pontomedullary and pontomesencephalic levels of the brain [26]. The child also underwent an EEG before the start of treatment and after 6 months.
The safety of intravenous CBC suspension was assessed on the basis of the Common Terminology Criteria for Adverse Events scale (CTCAE)1. In accordance with this scale, all adverse events are ranked as mild, moderate, severe, life-threatening, and resulting in death.
THERAPY RESULTS
CBCs at a dose of 250 ± 20 million cells were well tolerated. No adverse events were recorded during therapy.
Changes in the patient’s condition according to the psychometric and instrumental assessment
Before CBC administration, due to significant communication disorders and emotional-behavioral characteristics, it was not possible to conduct a complete standard examination using the Wechsler scale. Therefore, we selected separate subtests of the scale to assess the main cognitive functions. The “Digit span” subtest assessed working memory and active attention; the “Picture completion” subtest was used to assess perceptual abilities, observation, and concentration; the “Kohs blocks” subtest was used to assess analytical and synthetic abilities; and the “Coding” subtest helped assess attention characteristics and the development of hand-eye coordination. Test results are presented in Table 1.
Table 1. Results of the separate subtests of the Wechsler scale before the start of therapy and after 6 and 12 months
|
Subtest name |
Before the start of therapy |
After 6 months |
After 12 months |
|
Digit span (attention and memory) |
1 (attention — 0, memory — 0 points) |
2 (attention — 0, memory — 2 points) |
1 (attention — 0, memory — 2 points) |
|
Picture completion (perceptual ability, focus) |
0 |
0 |
5 |
|
Kohs blocks (analysis-synthesis with a visual standard) |
4 |
5 |
8 |
|
Coding (hand-eye coordination, speed of formation of new skills) |
2 |
4 |
9 |
Note: 0 — did not understand the instruction; normal range is above 5.
As can be seen from the presented table, there is a significant increase in the “Picture completion”, “Kohs blocks”, and “Coding” subtest scores. It should also be noted that 12 months after the first CBC administration, a full Wechsler test could be conducted with this patient. The verbal indicator of intelligence in formal numerical terms was 42 points, and the non-verbal was 79 points. Due to the large speard of the results it was not possible to obtain an overall Wechsler intelligence score. Testing became possible thanks to significant improvements in the contact, attention, and emotional-volitional control of the patient’s behavior.
Analysis of the CASD and ATEK scales also revealed a significant improvement (Tables 2 and 3). The total CASD score decreased from 16 (before the first injection) to 6 (6 months after the first injection), the total ATEC score decreased from 80 (before the first injection) to 16 (6 months after the first injection). The ATEC score decreased from 27 to 3 in the “Socialization section, from 23 to 7 in the “Health/growth development/behavior” section, from 18 to 5 in the “Speech/language/communication skills” section, and from 12 to 1 in the “Sensory skills/cognition” section. The greatest improvement of the CASD score was in the “Obsessive actions (perseveration)” section (a decrease from 4 [out of 5] to 0) and in the “Mood disorders” section (a decrease from 2 [out of 4] to 0).
Table 2. CASD score before and 6 months after the start of therapy
|
CASD sections |
Before the start of therapy |
After 6 months |
|
«Social interaction issues» |
1 |
1 |
|
«Perseverations» |
4 |
0 |
|
«Somatosensory disorders» |
2 |
1 |
|
«Deviations in communication and development» |
5 |
4 |
|
«Mood disorders» |
2 |
0 |
|
«Attention and danger awareness issues» |
2 |
0 |
|
Total score |
16 |
6 |
Table 3. ATEK score before and 6 months after the start of therapy
|
ATEK sections |
Before the start of therapy |
After 6 months |
|
Speech/Language/Communication Skills |
18 |
5 |
|
Socialization |
27 |
3 |
|
Sensory skills/Cognition |
12 |
1 |
|
Health/Growth development/Behavior |
23 |
7 |
|
Total score |
80 |
16 |
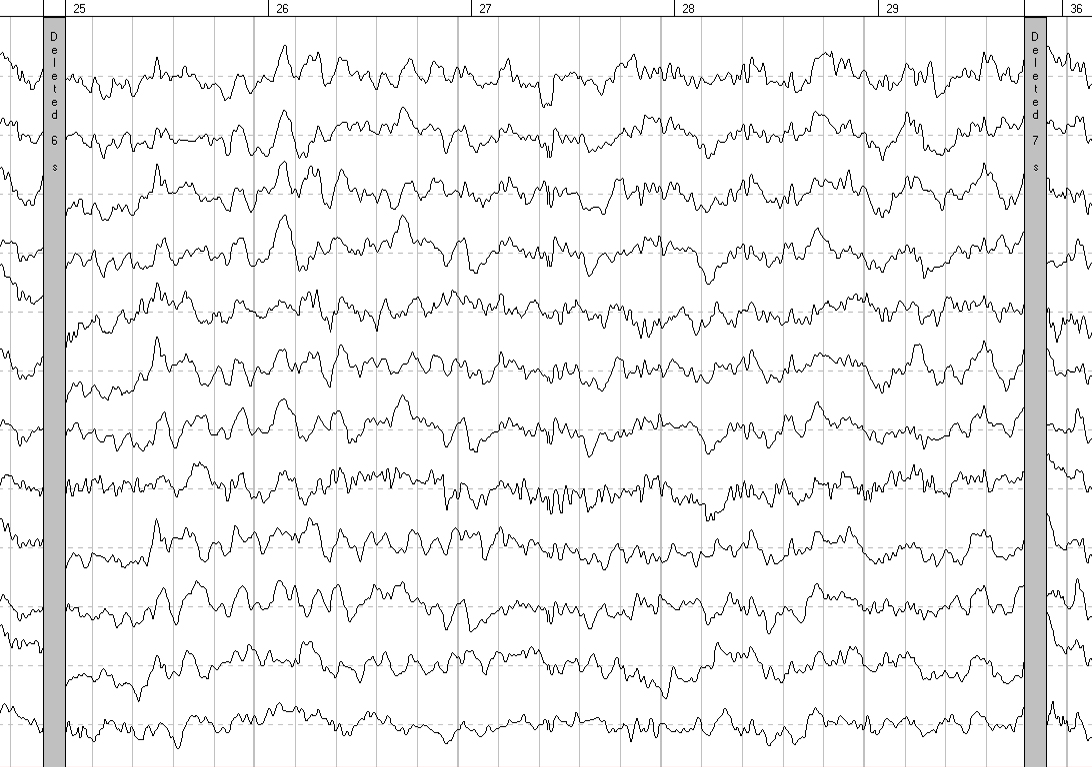
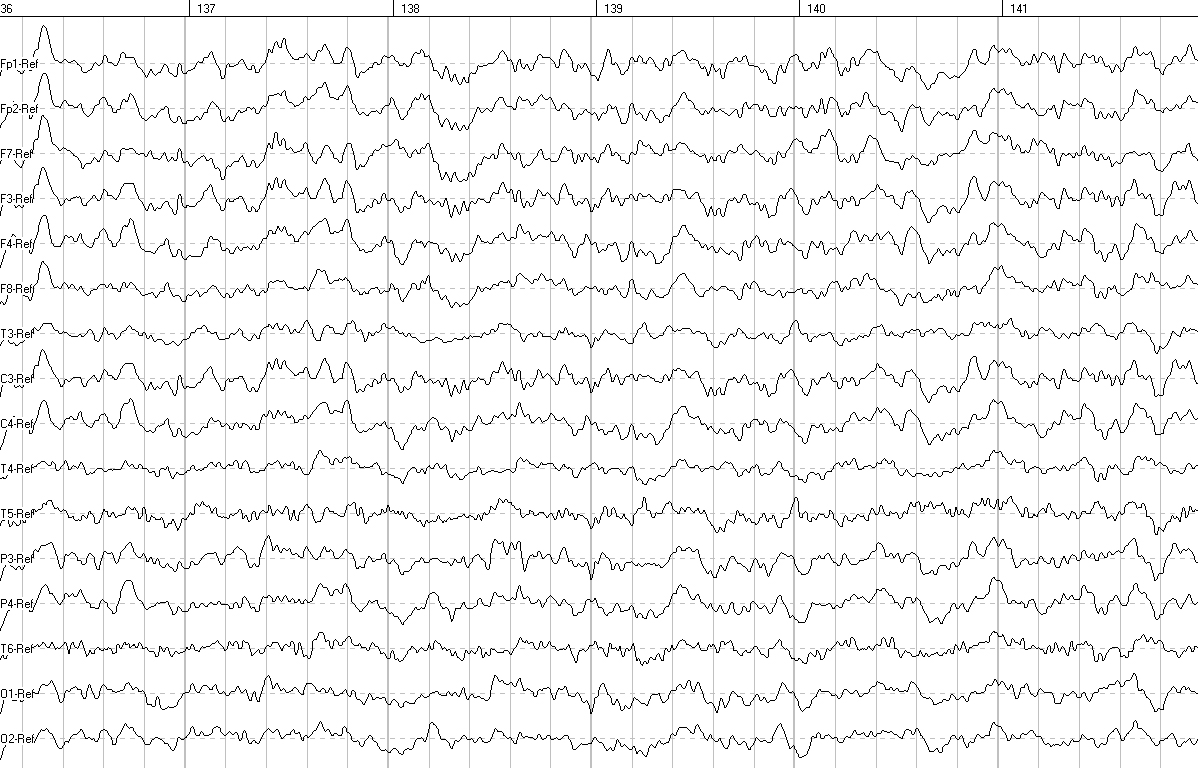
EEG analysis involved assessing the general functional state of the brain, the level of maturity of the bioelectrical activity, the severity of EEG changes, and clarifying the location of pathological changes; it showed a significant improvement of parameters 6 months after CBC administration (Figures 1, 2). BAEP analysis showed an improvement of auditory signal conduction in the “auditory olivary complex” area on the left 6 months after CBC administration (interval I-III: 2.57 ms before the start of therapy and 2.35 ms after 6 months).
Figure 1. EEG before the start of therapy
Figure 2. EEG 6 months after the start of therapy
EEG performed 6 months after the start of therapy showed an increase in the number of groups of α-waves, a decrease in the amplitude of the baseline EEG, a decrease in the number of polyphasic potentials in the occipital leads of both hemispheres, and a decrease in slow-wave spectrum waves (θ-waves, single σ-waves). There was also a significant decrease in amplitude characteristics and zoning in the form of a pronounced increase in amplitude from the frontal cortex to the occipital cortex, which was not observed before the start of therapy. Comparison of the quantitative EEG data with eyes closed shows the following: an increase in the power of α-waves in the parietal and occipital leads of both hemispheres and a decrease in the power of θ-waves in the occipital-parietal leads of both hemispheres. Analysis of the reorganization of the leading electroencephalographic pattern over time indicates a decrease in the severity of functional immaturity of the brain structures in the patient after treatment.
Changes in the patient’s condition revealed by clinical evaluation
A few months after the start of the therapy, the child’s vocabulary had expanded significantly, a simple phrase had appeared, and speech had begun to be communicative. But the most noticeable result was observed in the emotional dimension and behavior: the child became much calmer, aggression and affective outbursts disappeared, which made it possible to completely discontinue neuroleptics. The dramatic development of cognitive functions was reflected in the child’s drawings (Figures 3, 4). Before therapy, the patient’s drawings had been monotonous and non-objective. Thanks to the development of attention, perception, and fine motor skills, the child began to develop reading and writing skills.
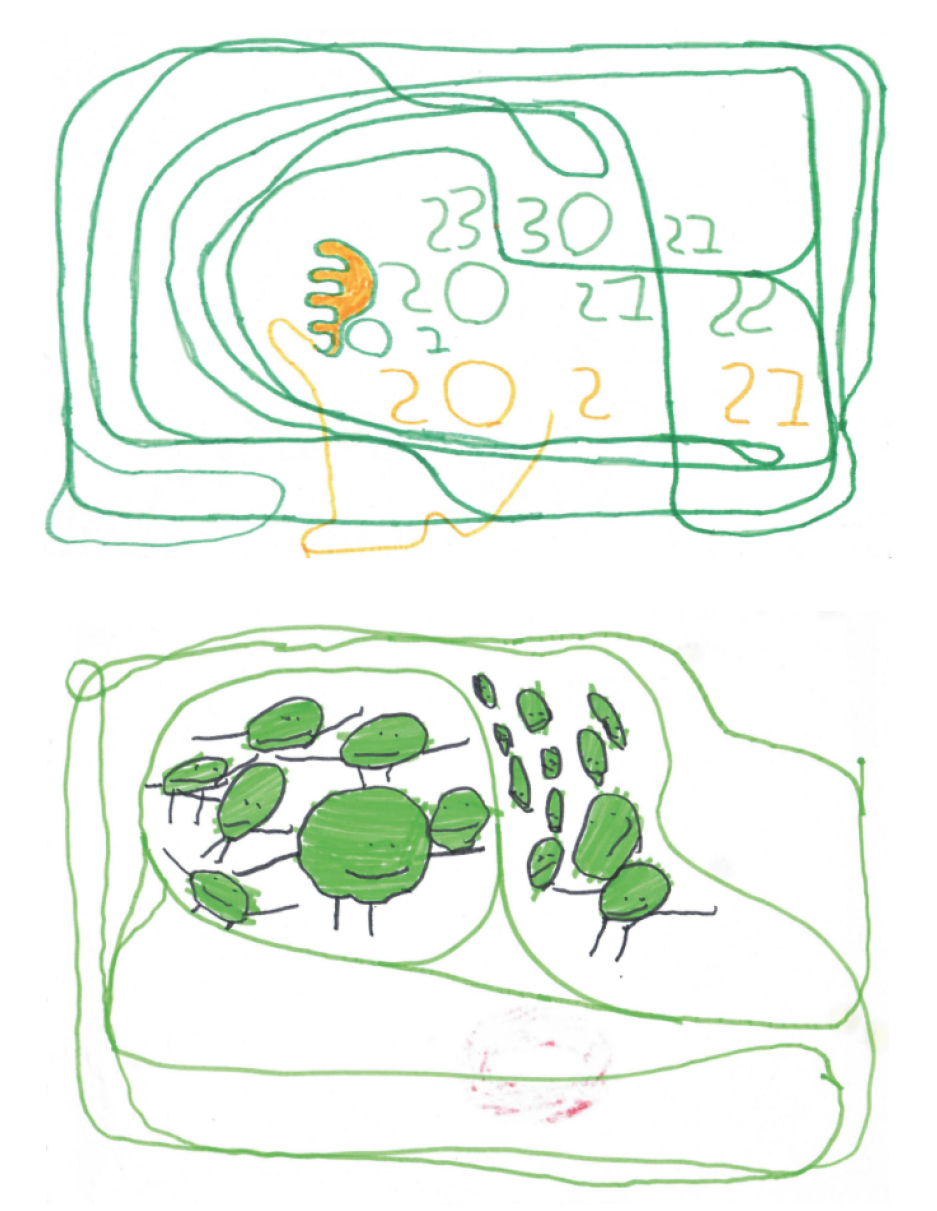
Figure 3. Patient’s drawings before the start of treatment
Figure 4. Patient’s drawings after 6–9 months
DISCUSSION
There has been a significant increase in research into the use of stem cells for the treatment of neurological and psychiatric disorders in recent decades. Among these, there are many case reports in which cell therapy produced a real breakthrough [27–31].
In our opinion, the same can be said about our case. Despite a large number of publications, there has not been a single successful case report of CBC therapy for regressive forms of autism, although there is a sufficient amount of data on the effect of neuroinflammation on the development of regressive autism, as well as on the immunomodulatory function of stem cells. Patients with regressive autism are characterized by immune disorders, increased production of pro-inflammatory cytokines, impaired blood-brain barrier, and subacute inflammation in the brain tissue [32]. Pro-inflammatory cytokines, by penetrating the blood-brain barrier, affect microglia and astroglia, which leads to impaired pruning and synaptic transmission [33]. From the microanatomical perspective, patients with autism have a shorter length of dendrites (especially in the frontal, temporal, and motor cortex), an increased number of cortical columns with a decrease in their volume, and blurred gray-white matter transition [34, 35]. The pro-inflammatory immune status of such patients causes a tendency toward an inadequate response of the immune system to triggers (infections, immunization) and the production of autoantibodies to the body’s own tissues, which often leads to a regression of previously acquired skills and an increase in autistic symptoms [36, 37]. This is also confirmed by studies on the successful use of anti-inflammatory therapy for the treatment of regressive autism [38, 39]. Numerous studies have shown that stem cells (SCs) have an immunomodulatory effect, suppressing the activity of innate immunity factors (dendritic cells, natural killer cells, complement) and the functions of cytotoxic T-lymphocytes and T-helper cells. In addition, SCs translate their functions to other cells, in particular, regulatory T-lymphocytes, which determines the effectiveness of cell therapy even after lysis of the injected stem cells [40–42]. The regression and the development of the disease two years after an infection suggest that our patient has a regressive form of autism, which is mostly characterized by immune disorders [32]. This assumption explains the significant improvement in the drug-resistant patient following CBC therapy. However, it should be noted that the described case is currently the only one and that confirmation of the hypothesis requires extended clinical studies on a large sample of patients with regressive forms of autism, as well as immunobiochemical studies confirming the abnormal immune status of their patients.
CONCLUSION
The use of CBCs is associated with good tolerability and the absence of significant adverse events. The use of nucleated CBCs in a patient with regressive autism led to a significant improvement in cognition and a decrease in the severity of their autistic symptoms. There was an improvement in the child’s condition in the form of perception development, reduction of somatosensory disorders, normalization of emotional status, and development of socialization and communication skills. The complete discontinuation of neuroleptics is also an important positive result of the treatment. This case provides evidence that the use of CBCs in some forms of autism in children can lead to a significant improvement in their condition.