In English
Introduction
Early life stress (ELS) events have the potential to adversely impact future life outcomes in regard to health and social well-being. Whereas evidence exists that there is a connection between ELS and later developmental outcomes, the mechanisms underlying them remain unrevealed. Studying ELS in the human population is complicated due to ethical reasons and the challenge of conducting research with human subjects. Therefore, it is important to develop studies that interrogate this connection in animal models to derive biological mechanisms that can be translated to the human population. There are numerous and diverse negative ELS effects that impact later development in mice that have been shown previously, such as deficits in working memory
[Rocha, 2021], telomere shortening
[Sarıbal, 2021; Rentscher, 2020], alterations in the structure and functioning of certain brain regions, including those related to the hypothalamic-pituitary-adrenal (HPA) axis—a major neuroendocrine system that controls reactions to stress and regulates many body processes
[Kotlinska, 2023; Trask, 2023], hyperactivation of the HPA axis in response to acute stress
[Wang, 2022], and alterations in DNA methylation of a neuron-specific glucocorticoid receptor (GR) gene Nr3c1
[Rahman, 2022; Rentscher, 2020], among others. GR, binding glucocorticoids (cortisol in humans and corticosterone in mice), is the primary mediator of feedback regulation in the HPA axis. These effects have also been registered in humans but only at the level of associations.
This study pursues several aims. First, it aims to create a new combination of behavioral tests and a wide range of biological markers that will allow for a comprehensive analysis and an enhanced understanding of the ELS outcomes at different levels (psychological, physiological, and genomic). All tests and biosample collections in this study are designed so that the results can be subsequently connected to the relevant human studies. Second, this study aims to improve our experimental design and establish a robust protocol by conducting a feasibility study before launching a larger-scale investigation. Such a step is important since all model studies involve animal sacrifice, and it is the investigator’s responsibility to reduce the number of animals used in research. Executing a feasibility evaluation of the protocol allows researchers to identify potential flaws, enabling timely adjustments and the more efficient utilization of animals. We believe that implementing this protocol on a full-sized cohort of animals will empower our search for mechanisms that are behind adverse outcomes of ELS at the level of epigenetics, physiology, cellular architecture of the brain, and structural elements of chromosomes, in addition to the behavioral outcomes. Thus, in this feasibility study, we model the causal effects of ELS exposure on multi-faceted aspects of mouse development to shed light on similar mechanisms of exposure to adverse childhood experiences in humans.
Materials and Methods
Animals
Animal housing was provided by the Animal Care Operations (ACO) department at the University of Houston (UH) in accordance with national laws and requirements for the care and use of animal subjects. All procedures performed with animals during this study are approved by the UH IACUC protocol PROTO202100004.
Laboratory-inbred C57Bl/6 mice (
Mus musculus) were used as the main subjects in this study. Pregnant nulliparous females were purchased from The Jackson Laboratory (Headquarters Bar Harbor, Maine, U.S.). Animals were delivered at the facility on GD15 (gestational day) and gave birth on GD19.5 according to established timelines
[Hasegawa, 2022]. Litter sizes were approximately 4-5 pups, which is slightly less than an average size of 6.6 pups per nest for this strain
[Fox, 1997]. In total, we collected 14 pups from three females.
Laboratory-inbred CD-1 mice (Mus musculus) were used as residents in the social defeat paradigm. Animals were purchased from Charles River Laboratories (Wilmington, Massachusetts, U.S.), and the strain was supported by breeding in the ACO department at the University of Houston. Only males were used in this experiment.
Upon arrival, female pregnant C57Bl/6 mice were housed individually in a male-free colony room. Mice were kept individually in standard polypropylene ventilated cages (IVC) (Optimice®) with a solid floor, under controlled temperature (21°C) and humidity (27%) with 12/12 h light-dark cycle (lights on at 7 am). Animals had access to bedding and nesting material; water and food (standard rodent chow) were provided ad libitum. Pregnant dams were checked daily, and the day of parturition was designated as postnatal day (PND) zero, PND0. It can be challenging to determine the exact day of birth if cages are not checked every other hour; for example, one may miss the birth of the litter if it is only checked once in the morning. It is more suitable to check mice several times a day to accurately stage mouse pups. Additionally, to clearly distinguish between PND0 and PND1, the following indicators were used: to label pups as PND0, we observed the presence of blood and placental fragments on the bedding and whether pups were scattered throughout the cage. To label pups as PND1, we assessed if pups had already been retrieved to the nest and that the bedding was free from blood and placental fragments. After delivery, one of the dams was assigned to the control group, and two dams were assigned to the MS group.
Maternal separation
The MS is a commonly used model of ELS. For this experiment, the MS procedure was designed based on an established protocol
[van Heerden, 2010], with the difference being the time at which it was performed. Although various periods of the day can be used for separation, morning hours might be preferable in rodents due to circadian fluctuations in stress hormones and associated basal levels of anxiety and stress. MS itself causes stress, especially a long one, so it is recommended to perform it in the morning hours when the basal level of stress is lowest in mice
[Razzoli, 2011; van Heerden, 2010]. For this procedure, two adjoining rooms were used to avoid additional stress on the animals from being transported through the hall and encountering visual, olfactory, and auditory stimuli. Only investigators involved in this experiment and technical staff were allowed to enter both rooms, trying to keep the number of visits to a minimum.
Starting at PND3 to PND14, MS litters were separated from randomized dams for three hours per day, starting at 9:00 am and ending at 12:00 pm. First, dams were removed from the home cage and placed in a novel ‘temporary’ cage (regular IVC) with fresh bedding and nesting material, food, and water. Then, the ‘temporary’ cage was taken to the adjoining room to exclude olfactory or ultrasound vocalization exchanges between dams and their pups. After dams were taken, pups were individually placed in novel polypropylene boxes with fresh bedding separated from their littermates. Boxes were placed on a heating pad maintained at 37℃. Photos of pups were taken daily with the flash turned off. These visual materials were used to quantify animal general development based on such milestones as eye-opening and hair growth
[MacDowell C.J, 2024]. After three hours, pups were placed back in their home cages without disturbing the nest. To complete the procedure, dams were returned to their home cages. During the whole separation period (PND3—PND14), the same ‘temporary’ cages were used. In the control group, pups were left undisturbed with their dams until weaning at PND21, with the exception of routine cage cleaning. Cages were cleaned once in two weeks, and for MS families, nesting material was changed at PND2, PND9, and PND16. At PND21, all pups were weaned and housed in groups of 3—5 per cage. At PND28, pups received ear tags. Starting from PND31 to PND36, pups were weighed daily; starting from PND34, all mice were food-restricted to 70% of the daily ratio.
Behavioral assessments
The MS protocol was followed by the radial maze test and social defeat (SD) test to study the working memory and self-regulation of the stressed as compared to unstressed animals. Although the mouse model of SD includes a physical confrontation component, its psychological component has some similarities with receiving social evaluative threats from the Trier Social Stress Test performed in humans
[Stengel, 2011]. The eight-arm radial maze was used to evaluate working memory, and the SD paradigm was used to assess the stress reactivity. Behavioral tests were performed from least invasive (the maze) to most invasive (SD), separated by 6—7 days to allow the animals to recover. All tests were videotaped for subsequent behavioral scoring using ANY-maze camera and software (Stoelting Co, USA). Behavioral testing was conducted starting at 9:00 am and ending at 12:00 pm. All animals were taken to the testing room one hour before the beginning of testing and allowed to recover after transportation. A soundproof, ventilated cabinet with daylighting (Med Associates Inc, USA) was installed in the room to isolate waiting animals from the test subjects and to minimize the auditory and visual stimulation they received. Blood and brain collection was performed on the same day as the SD paradigm (PND46 and PND47, four mice each day).
Radial arm maze test
A working memory test was performed using the eight-arm radial maze protocol
[Stanojevic, 2022] with modifications. First, the habituation phase took place for one day instead of two, and second, there were no learning days. A one-day habituation phase is considered sufficient time for rodents to become familiar with the new apparatus and save time
[Kotlinska, 2023; Whitaker, 2007]. Learning days were eliminated as the original paradigm used by Stanojevic et al. (ref) was applied to studying long-term memory, and, therefore, animals needed to be trained; this training was needed for our experiment, as we studied working memory.
The apparatus consists of eight identical arms (5 cm width, 35 cm length, 9 cm height) extending radially from an octagonal platform (Stoelting Co, USA). A camera was placed right above the apparatus to record the experiment. The test was performed in two phases: a habituation phase on Day 1 (PND38), which consisted of one exploratory trial that lasted ten minutes to prepare animals for the maze where the food pellets were placed at the end and entrance of all eight arms. The main phase at PND39 consisted of one trial that lasted five minutes, where one food pellet was placed at the end of an arm (once selected at random); the arm and the location of the pellet were fixed for all mice. In each main phase, the mouse was placed on the central platform and allowed to move freely. An arm entry was counted when all four animal paws crossed the entrance of the arm. Re-entry in a previously visited baited arm is considered a working memory error. After the completion of each test, the apparatus was cleaned with 70% Ethanol.
Social defeat paradigm
A resident-intruder test was performed according to a previously developed protocol
[Razzoli, 2011] with the difference that, in our study, the procedure was carried out once, without repetition, since we aimed to create acute stress. C57Bl/6 mice at PND46 and PND47, weighing 16—21 g, served as experimental subjects. CD-1 male mice, which are distinctly larger than experimental subjects, served as residents. Residents were housed singularly. C57BL/6 mice were introduced into the home cage of an unfamiliar CD-1 male mouse for a 10-minute interaction. Each CD-1 male was subsequently used two times with two different C57Bl/6 mice before being replaced by the next resident. Each CD-1 mouse performed only two times a day. After the interaction, the mouse was returned to its home cage.
Urine collecting for corticosterone measurement
Before interacting with the CD-1 mouse, each C57Bl/6 mouse was placed in a novel transparent polypropylene box without bedding material for 10 minutes. This was enough time for a mouse to urinate. Urine was collected into a 1.5ml tube. The same was done after the interaction. The box was cleaned between sample collections. All samples were placed on ice right after being collected and then placed for long storage.
Blood collecting for methylation profiling
Blood was collected using intracardiac puncture as a terminal procedure. The mouse was anesthetized using an isoflurane vaporizer (SomnoSuite, Kent Scientific) and placed on its back. After opening the chest, a 22-gauge needle was inserted slightly to the left at the base of the sternum, directed toward the animal's head and parallel to the table. Blood was collected in 2ml tubes with Ethylene Diamine Tetra Acetic acid (EDTA) to prevent blood samples from clotting.
Brain sectioning for methylation profiling and telomere length analysis
Mice were decapitated using scissors right after blood collection. The skull was opened, and the brain was quickly removed and placed in a cooled Stainless-Steel Coronal Brain Matrix (Kent Scientific). The Matrix was placed on a cold metal block to maintain a low temperature. All instruments were also cooled before the procedure. The brain was rapidly washed in cold 1X RNase-free Phosphate-Buffered Saline, PBS (Hygia Reagents, San Diego, USA), and three sections were cut out. Based on the map designed by Heffner et al.
[Heffner, 1980], we took sections 7—8 for the hippocampus, 5—6 for the amygdala, and 2—3 for the prefrontal cortex. The hippocampus and amygdala tissues were cut from the corresponding sections; for the prefrontal cortex, the whole section was taken. All brain tissues were rapidly collected in 1.5ml tubes, immediately placed on ice, and then stored at -80°C until subsequent processing. Brains from 4 mice were sectioned.
Brain dissection for immunohistochemistry (IHC)
After blood collection, transcardiac perfusion was performed according to the protocol designed by Wu and colleagues
[Wu, 2021]. We followed this protocol starting from the “Transcardiac perfusion with saline” without any modifications, so, therefore, we refrain from describing the procedure in detail here. After brain samples were fixed in 4% PFA, they were dehydrated by being placed in 70% Ethanol for 30 min, then placed in 90% Ethanol for 30 min, and finally placed in 100% Ethanol for 1 hour. After dehydration in alcohol, brain samples were placed in an Xylene Substitute (Sigma-Aldrich) for 90 min and then placed in melted paraffin for 4 hours. Finally, samples were infused with melted wax and left to dry. Brains from 4 mice were processed for subsequent IHC analysis.
Results
In this feasibility study, we designed and executed a protocol for the evaluation of cognitive abilities, specifically working memory, and social behavior, specifically social interaction, in mice following ELS due to repeated maternal separation (Figure 1).
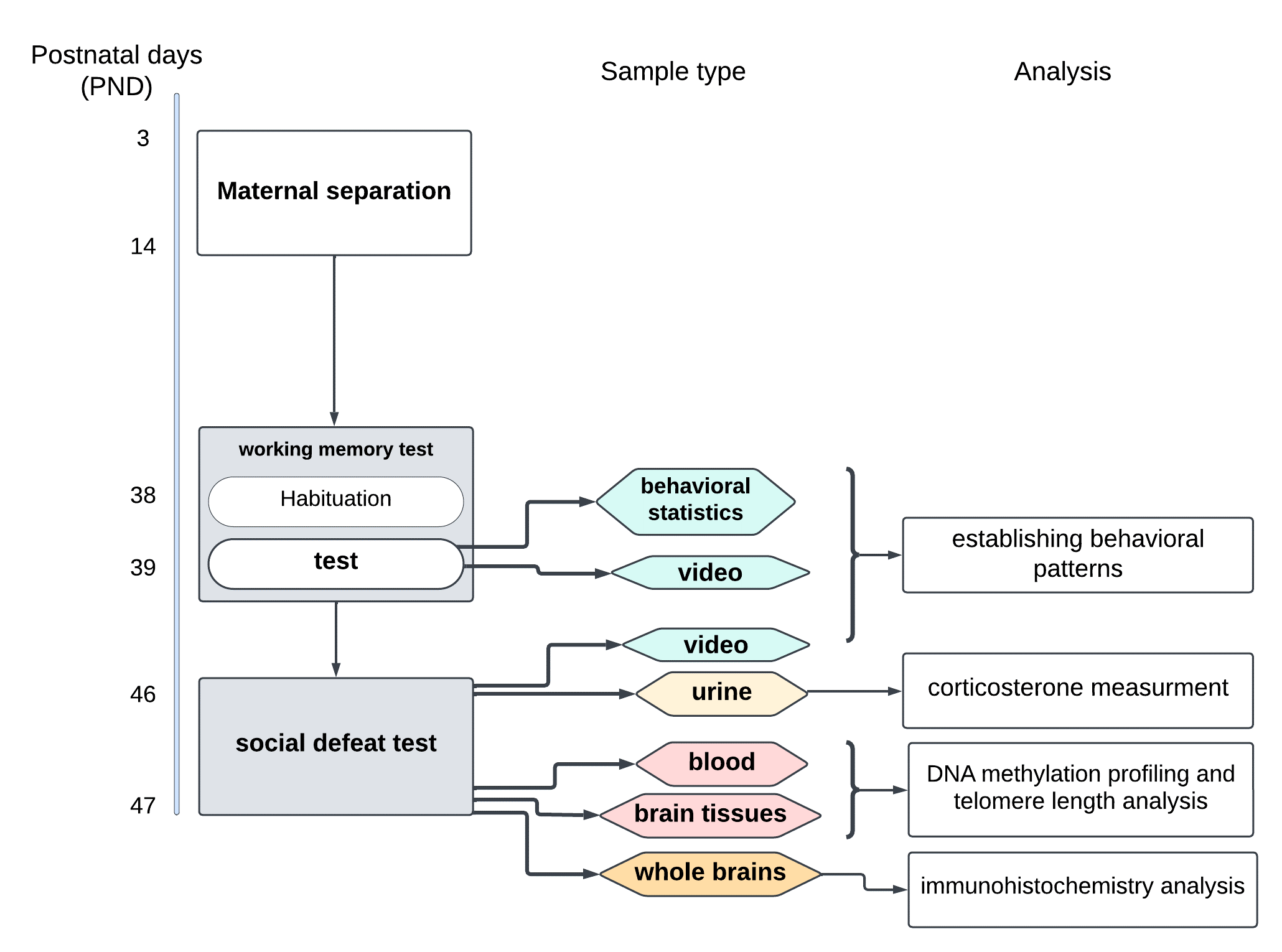
Fig. 1. Workflow diagram
The entire protocol took 49 days from delivery to sacrifice. Fourteen pups from three families entered the protocol. On PND5, one pup was lost due to maternal cannibalism. Female rodents are known to eat their litter due to various reasons
[Brajon, 2021]. On PND20, one pup died in the MS1 nest and was removed from the cage. Pups in both MS nests developed slower than normal, according to the Jackson laboratory’s visual materials on typical development (see https://oacu.oir.nih.gov/system/files/media/file/2021-02/jaxpupsposter.pdf). For example, eyes were supposed to open on PND11-12, but in our experimental group, it occurred on PND20. Unfortunately, during the separation stage of the experiment, we could not compare pups in the MS nests with pups in the control nest since the litter had to remain undisturbed until PND21. Visual analysis performed on the day of weaning (PND21) showed that pups in the control nest were more active and larger in size compared to pups in both MS nests. On PND27, four more pups died, one from the MS2 nest and three from the MS1 nest; thus, the whole litter from the MS1 nest was lost. For the C57BL/6 strain, 76.6% is the average survival rate before weaning in the absence of stress
[Whitaker, 2007], while our average rate for both groups is 53.3%. The mortality rate we encountered was unexpectedly high. Perhaps this can be explained by the prenatal stress experienced by the mother during extended transportation from the vendor to our ACO facility. In the Discussion, we propose several options for solving transportation problems.
Due to the losses incurred, at the beginning of the behavioral experiments, the MS group consisted of three males and the control group consisted of 4 females and one male. Pups in both groups were underweight according to the weight data provided by the Jackson Laboratory (Table 1). The radial maze sessions were performed with eight animals. Mice in both groups re-entered the baited arm during the main trial, which means they conducted a memory error. However, a larger number of animals in both groups is needed to carry out adequately powered statistical analyses.
Table 1.
Weight gain dynamics. Average weight ± standard deviation is shown, with values for the single male excluded in the control group. Values for the “Jackson Lab” columns were taken from the vendor’s website (https://www.jax.org/jax-mice-and-services/strain-data-sheet-pages/body-weight-chart-000664).
|
PND
|
Females (grams; ± st.dev)
|
Males (grams; ± st.dev)
|
|
|
Control nest
|
Jackson Lab
|
MS nest
|
Jackson Lab
|
|
31
|
12.5 ± 1.29
|
14.7 ± 1.8
|
11.7 ± 3.5
|
16.5 ± 2.6
|
|
35
|
13.5 ± 2.1
|
17.8 ± 1.1
|
13.3 ± 2.5
|
20.7 ± 1.8
|
|
47
|
17 ± 0.58
|
18.75 ± 0.95
|
17.7 ± 2.1
|
22.75 ± 1.65
|
Using ANY-maze software allowed us to obtain a wide range of data, such as the number of entries in each arm, the duration of the visits, and various behavior reactions such as freezing, immobilization, and escape attempts. Thus, in addition to assessing working memory, complex behavioral patterns may be analyzed based on the data obtained from this test. Despite the small sample size, a variety of individual behavior patterns were noted. For example, some mice actively explored the maze, while others were not eager to engage in any explorations. And there was one animal that regularly tried to escape by jumping out. The SD test was performed with four animals: one male and one female were randomly selected from the control, and two males were selected from the MS group. Although there were single acts of aggression (biting) demonstrated by resident CD-1 mice, all C57BL/6 animals showed no signs of subordination. After the defeat, typical behavior includes sideways or upright submissive postures, withdrawal, fleeing, lying on the back, or freezing
[Murra, 2022]. We recorded chasing, olfactory contacts, and single bites in experimental animals when the resident demonstrated high-quality aggressive bouts (defined as repetitive attacks
[Golden, 2011]). The social defeat paradigm is a well-established behavioral test, yet its implementation is challenging. To successfully conduct the SD paradigm, many specific details must be taken into account. Importantly, in this feasibility study, we observed details presented in the Discussion that need to be considered in the main study.
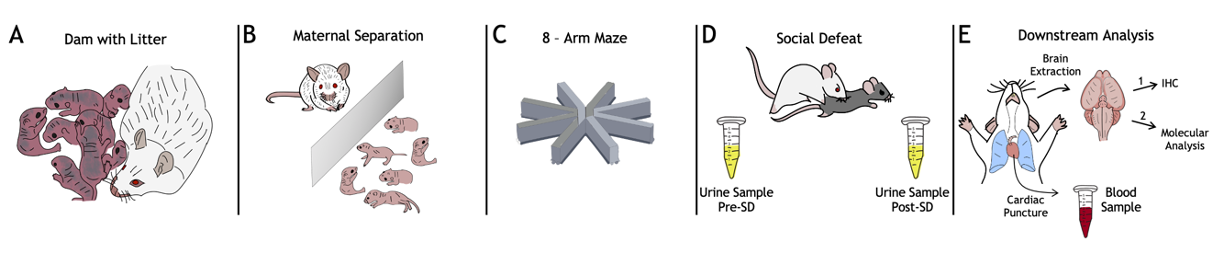
Fig. 2. The experiment roadmap. A) Dam with litter. At this stage, the number of pups in a litter is evaluated; litters with less than 3 pups are excluded from the protocol; B) During the MS, developmental milestones (eyes opening, ear positioning, and hair growth) are tracked; C) In radial maze test the number of re-entries in the baited arm for each animal is counted; D) In SD test the number and duration of attacks is noted, as well as specific submissive poses. Urine samples are collected and processed for the enzyme-linked immunosorbent assay (ELISA) to measure the amount of corticosterone in a sample; the ELISA is performed according to the protocol described in
[Kim, 2018]; E) Blood sample is collected using cardiac puncture for subsequent DNA extraction followed by telomere length analysis using a quantitative real-time PCR (the protocol is described in
[Sarıbal, 2021]), and epigenotyping via genome-wide DNA methylation array. Prior to the brain tissue collection, the MS and control animals are randomly assigned to two groups for 2 different downstream analyses of the target brain regions — amygdala, hippocampus, and prefrontal cortex: (1) immunohistochemical analysis (see Methods,
[Wu, 2021]) of GR density according to the manual described in
[Stanojevic, 2022], and (2) DNA extraction followed by the telomere length measurement and DNA methylation profiling using the above molecular genetic techniques.
The SD test was followed by urine collection. Urine samples will be used for corticosterone measurement using the enzyme-linked immunosorbent assay (ELISA) method. After the SD test mice were sacrificed, the blood and brain specimens were collected. Blood samples were frozen; they will be used for DNA extraction. Brains were distributed into 2 sets for different types of analysis: the first set included 4 whole brains (2 from MS mice and 2 from the control group) and was allocated for IHC analysis. The second set also included 4 brains (1 from MS and 3 from control mice) and was assigned for DNA extraction. DNA from both blood and brain will be used, first for telomere length analysis using the PCR method and second - for epigenotyping, so we will get methylation patterns for subsequent analysis. A full analytical plan for different types of data is shown in Figure 2.
We did not experience data loss in this study, but this may be due to the small number of animals. In larger studies, data loss could occur due to various reasons in each of the tests, taking into account the number of different types of data collected (i.e., behavioral, hormonal, (epi)genetic, and neurobiological).
Discussion
In this study, we designed and evaluated the feasibility of an experimental protocol that combines classic tests to study the behavioral outcomes of ELS with modern methods of molecular analysis to reveal possible mechanisms underlying these outcomes at different levels: hormonal changes, DNA methylation patterns, cellular architecture of the brain, and structural elements of chromosomes. While most research in this area focuses on specific targeted changes that occur because of ELS, we propose a protocol for a comprehensive analysis combining multiple tests and biomaterials that potentially respond to MS. The utilization of a combination of tests, previously used individually or in other frameworks, can lead to new insights. While all elements of this protocol have been used in previous publications
[Razzoli, 2011; van Heerden, 2010; Stanojevic, 2022], we believe that this particular combination of tests is unique and, therefore, valuable.
In our study, mice repeatedly visited the baited arm in the maze test, which indicates deficits in working memory and self-regulation. Working memory is known to share an overlapping neural circuit with the HPA response to psychological stress
[Lin, 2020], and ELS is one of the reasons for alterations in HPA functioning. In search of the molecular signatures of these alterations, we are planning to study methylation profiles of genes involved in the HPA development and functioning. For that purpose, we will use DNA extracted from the blood and brain samples of MS and non-MS mice. One of the target genes is
Nr3c1, which codes the GR receptor that binds corticosterone. We plan to study not only the
Nr3c1 methylation pattern but also the GR receptor’s density in brain structures related to HPA. If a reduced density is observed, it could explain the persistence of corticosterone levels in response to acute stress, which was shown for rodents after ELS
[Bonapersona, 2019]. Corticosterone levels will be evaluated as well using the urine samples collected after the SD test.
One of the aims of this study was to determine potential flaws in the designed paradigm and its implementation and perform the needed troubleshooting. We have encountered several difficulties that we believe are worth discussing so that they can be successfully resolved. First, we noticed a slightly reduced body weight and survival rate of pups in both the MS and control groups. Reduced birth weight has been shown to be one of the outcomes of prenatal stress during the second half of pregnancy in rodents
[Possamai-Della, 2023]. While the maternal HPA axis response to stress is significantly attenuated during the second half of pregnancy
[Possamai-Della, 2023], the stress in later gestation stages in mice still could have led to undesirable consequences. In our case, all three pregnant dams were transported from the vendor to the ACO facility at 13-15 gestation days, which is almost the end of pregnancy (it usually lasts 19.5 days) for the C57BL/6 strain. The vendor experienced an unexpected technical delivery delay that might have caused extra stress for the dams. Although such technical problems cannot be avoided entirely, we would suggest ordering dams at 8-9 days of gestation, approximately in the middle of pregnancy, and requesting that mice are not transported on weekends or holidays to avoid additional travel time. Maintaining a mice colony in the local ACO facility may also be a solution to this problem. Reduced birth weight could also explain the increased mortality of the pups across all nests. Perhaps in the future, it would be worth excluding from the protocol the pregnant dams that experienced problematic delivery. However, if such mortality is observed during the experiment and it is not related to housing conditions, then little can be done. Switching to a less susceptible mouse strain may be an option.
The second difficulty concerns the social defeat paradigm that we performed. As recommended
[Golden, 2011], larger CD-1 resident mice were selected in comparison to intruder C57BL/6 mice. However, the size dominance was not sufficient to challenge the experimental mice. In the future, we plan to introduce several additions to the current version of the protocol. First, we will preliminarily select CD-1 mice with consistent levels of aggressive behavior and of a much older age than C57BL/6 mice
[Golden, 2011]. Next, an additional variation of the SD test designed for female mice will be introduced to our study. The classical version of the SD paradigm includes only males, as they tend to demonstrate aggressive behavior, but our aim is to acquire data on the ELS-driven social behavior changes for both males and females. Today, several modifications have been developed for the SD protocol, as the need to include females in rodent social stress models is growing. Instead of an adult CD-1 male, a retired breeder adult male can be used as a resident for encountering a female intruder. A retired male is less likely to perform mating behavior and more likely to attack a female
[Razzoli, 2011]. Lactating females could also be used as residents
[Holly, 2012; Jacobson-Pick, 2013; Ródenas-González, 2023], as well as females housed with castrated males
[Newman, 2019].
Third, in our initial experimental design, we selected urine collection over peripheral blood, as both bioliquids have been successfully used for corticosterone measurement
[Kim, 2018; Touma, 2003], and urinary and serum corticosterone levels have been shown to correlate
[Thorpe, 2012]. In our future experiments, urine collection will be used as a less invasive procedure that permits repeated sampling without causing extra stress. Moreover, during this feasibility study, a baseline for corticosterone was not measured, which will be carried out in a full-fledged study.
Of note is that this study does not present the statistical analysis of the data. This is due to the following: (1) there was a small number of animals in each group (3 and 5 mice) and (2) these groups were not sex-balanced. These limitations will be resolved in a study with a larger number of animals, which will also allow us to connect the data more adequately with human studies involving participants of both sexes. For the full-fledged study, a between-group comparison of animals with and without MS will be performed in terms of their learning, social behavior, and biological markers, taking the sexes into account
[Krauth, 1983].
Yet, the successful execution of this feasibility study demonstrates that this protocol can be transferred to a larger-scale project while maintaining the timeline. The feasibility study took 49 days from delivery to sacrifice. According to our estimates, an optimal workflow includes 4 litters (up to 24 pups) simultaneously, so scaling for larger studies may unfold as follows:
- While the first set of 4 litters undergoes MS, an order for 4 pregnant dams should be placed so they are delivered near the end of MS.
- When the first set is weaned and left to grow (for approximately 2 weeks), the second set of 4 litters is ready for MS.
- When the second set of litters is weaned and left to grow, the first set can perform behavioral experiments. Thus, the researchers have two weeks to conduct the maze test and the SD test and to sacrifice animals. Having completed this first round, they can engage the second set of mice. As a result, nine weeks are needed to process approximately 50 mice. This number can be increased if there are multiple rooms for MS, behavior rooms, and enough personnel.
Conclusion
To date, numerous animal protocols have been developed to study the consequences of ELS (primarily, MS), most of which focus on specific outcomes, such as changes in DNA methylation profile
[Rahman, 2022; Smith, 2020] or brain anatomy
[Hisey, 2023]. We believe that the proposed integrated approach combining a broad range of behavioral and molecular tests has a greater, compared to fragmented protocols, potential to uncover mechanisms underlying the effects of ELS and can significantly improve our understanding of the connection between ELS and later developmental outcomes.

