INTRODUCTION
Anhedonia is characterized by a decrease in or complete loss of the ability not only to consume positive emotions and interest in response to a stimulus (consummatory anhedonia), but also to anticipate potential rewards (anticipatory anhedonia), as well as the awareness of rewards [1]. This phenomenon is considered to be a symptom of regulatory disruptions in the brain reward system [2]. Anhedonia has a transdiagnostic nature and is one of the key symptoms of major depression, bipolar disorder (BD), schizophrenia, and addictions affecting the effectiveness of therapy and the clinical course [3, 4]. The role of anhedonia in the risk of suicidal behavior is highlighted, regardless of the severity of major depression [5].
According to the Research Domain Criteria (RDoC) approach, anhedonia can be considered as a dimensional trait, acting not only as a sign of psychopathology, but also as a characteristic of the reward system malfunctioning in individuals without mental disorders [6]. Consistently, healthy first-degree relatives of patients with major depression have a blunted reward sensitivity [7]. Therefore, the mechanisms associated with the development of anhedonia are often considered as candidates for the endophenotypes of major depression and other mental disorders [8, 9].
Dysfunction of the mesolimbic dopamine system and its interaction with the endogenous opioid system have been proposed as the central mechanism underlying anhedonia [10, 11]. Anhedonia is also associated with a decrease in volume and a change in functional activity in the medial frontal cortex and subcortical striatal areas (caudate nucleus and putamen) [12, 13]. There are studies of anhedonia in patients with somatic diseases, but their number remains extremely small [14–17].
Despite advances in biochemistry and neuroimaging, the genetic nature of anhedonia remains not fully understood. A study of 759 patients with depression revealed 18 single nucleotide polymorphisms (SNPs) that are associated with anhedonia [18]. A mega-analysis of three studies of young people from the UK and Sweden with a total sample size of 6,579 revealed one locus that was associated with anhedonia in the test sample, but not in the replication sample [19]. In a Finnish study, genetic associations with physical and social anhedonia were studied in 3,820 people, but no significant loci that reached a genome-wide significance level were identified [20].
In the largest genome-wide association study (GWAS) of anhedonia in the UK Biobank cohort (n=375,275), 11 new loci associated with anhedonia were identified with an SNP-based heritability score of 5.6% [21]. Strong positive genetic correlations were found between anhedonia and major depression, schizophrenia, and BD, but not with obsessive-compulsive disorder or Parkinson’s disease. Moreover, it was found that the genetic risk of anhedonia is associated with structures associated with the processing of reward and pleasure [21].
An important limitation of the GWAS studies is the use of phenotyping methods that evaluate anhedonia only at the current moment, and not during life (lifetime phenotype) [18–21]. This fact increases the risk of false negative responses and bias of the results, because a person with a certain genetic risk could have experienced anhedonia in the past, and not at the time of inclusion in the study. The authors however admit that people prone to anhedonia are more likely to report its manifestations at any given time, and that the “residual” phenotype of anhedonia will occur in people with a stronger genetic predisposition [21].
The aim of our study is to evaluate the genetic architecture of anhedonia and its overlap with other disorders.
Here, we present the first GWAS of the lifetime anhedonia phenotype in the Russian population, based on the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) criteria for anhedonia within the framework of major depression. Additionally, we perform polygenic risk scoring with summary statistics from a published large-scale GWAS to investigate the possible associations of anhedonia with various somatic conditions and mental disorders.
METHODS
Study design
This cross-sectional study was conducted under the auspices of the Russian National Consortium for Psychiatric Genetics [22]. The study was approved by an independent ethical committee in V.M. Bekhterev National Medical Research Centre for Psychiatry and Neurology (protocol No. 7 from 22.06.2017) and by the Genotek Ltd. ethics committee (protocol No. 12 from 26.10.2019). All procedures were performed in accordance with the World Medical Association Declaration of Helsinki. All participants signed a consent to the processing of personal data before registration.
Setting
The participants were recruited continuously amongst the clients of the Russian private genetic company Genotek Ltd. Most clients contact the company to determine their genotype in order to clarify their ethnic origin, seek dietary recommendations, and enquire about predispositions to various talents or health conditions. All subjects participated voluntarily and provided their genetic information for the study. They also completed an online questionnaire with socio-demographic and medical information posted on the Genotek Ltd. website1. The data was collected during 2019–2020. The data analysis was performed in 2021–2022.
Participants
The study involved respondents over 18 years of age, both sexes, height from 140 to 220 cm and weight from 40 to 150 kg.
Individuals who did not meet the stated age criteria (under 18 years of age), having abnormal height and weight (beyond 140–220 cm and 40–150 kg, respectively), as well as individuals whose biological samples did not pass quality control, were excluded from the study. Of the remaining 5,795 participants, only 5,724 completed the online survey questionnaire. In addition, all pairs of close relatives (up to 3 degrees of kinship) were identified based on genetic data using PRIMUS 16 and were excluded from the study. Of the remaining 5,116 participants, 4,520 passed the GWAS quality control test (for details see Section Genotyping).
Procedures
Phenotyping
Phenotyping of the participants took place on the Internet using an original screening test based on DSM-5 diagnostic criteria for depressive and generalized anxiety disorders [23]. The phenotype of anhedonia was determined in the study participants using a question based on the DSM-5 criteria for anhedonia in the framework of major depressive disorder: “Did you have a period (2 weeks or more) during which you received much less pleasure from what caused pleasure earlier?” According to the results of the answers (yes or no), the participants were stratified by the presence or absence of the lifetime anhedonia phenotype, respectively.
Genotyping
The DNA sample was obtained from saliva, and genotyping was performed using the Illumina Infinium Global Screening Array (GSA). Genetic data was subjected to quality filtering. We eliminated samples with genetic and reported sex mismatches, low call rate (<0.98), and abnormal heterozygosity (>3 standard deviations, based on linkage disequilibrium [LD]-pruned variants). Only good-quality DNA variants were retained for the analysis using the Hardy-Weinberg equilibrium filter (pHWE >1x10-5), call rate (>0.98), and minor allele frequency (MAF >0.01). Genotype imputation was performed using the Haplotype Reference Consortium (HRC) and 1000 Genomes reference panels using Beagle 5.1 [24–26]. Imputed variants with dosage R-squared DR2 >0.7 were kept for the downstream analysis. Thus, the quality control was conducted according to modern criteria [27].
GWAS methodology
GWAS analysis was performed with PLINK 1.9 [28]. We employed a logistical regression model corrected for age, sex, and the first 10 principal components (Figure S1 in the Supplementary). The Manhattan and Q-Q plots were built using the library “qqman” in R.
Prior to the GWAS analysis, population stratification was assessed and outliers were eliminated. At the first step, the Multidimensional Scaling (MDS) algorithm was employed for the Russian cohort, combined with the East Asian (EAS), African (AFR), and European (EUR) subsamples of 1000 Genomes. Common SNPs were used for both datasets, after filtering for HWE and LD pruning with the parameters (window=50 SNPs, R2 between SNPs <0.2). Based on the values of the first and second principal components, clustering was conducted using the Density-Based Spatial Clustering of Applications with the Noise algorithm [29]. Samples that did not fall within the clusters were excluded. After eliminating outliers, the MDS algorithm was re-applied (without combination with a subsample of 1000 Genomes). The first 15 components were later used as covariates to account for population stratification.
LD-blocks were defined based on SNPs with R2 >0.7 using the “LDPair Tool”, NIH, USA2. A single variant with minimal p was selected within each of these blocks, resulting in a total of 5 leading non-linked variants. The variants were annotated with SnpEff 4.3t [29], and additional information on each variant, including estimated allele frequencies (EAF), was obtained with the Database for Single Nucleotide Polymorphisms (NIH, USA3). Gene annotation was performed using GeneCards (Weizmann Institute of Science, Israel4). The methods are also described in our earlier article with the results of the Mendelian randomization analysis [30].
In addition, we used ENSEMBL POSTGAP5 to annotate variants with p <1x10-5 to the nearest genes. To find anatomical therapeutic chemical (ATC) categories enriched in the obtained gene list, we assembled a dataset of 1,716 gene-targets belonging to drugs from the 384 ATC categories present in DrugBank and performed a gene-set enrichment analysis using the package enrichR6. The package ABAEnrichment [31] was used to perform enrichment analysis across brain regions represented in the adult human brain transcriptome dataset from the Allen Brain Atlas database [32]. Counts of significant enrichments were visualized with the Coldcuts package (a subset of regions present in the Coldcuts segmentation was considered). The expression levels of each of the genes were obtained from the atlas for comparison. The pipeline of enrichment analyses used in the study is presented in Figure S2, A and B in the Supplementary.
SNP-based heritability
SNP-based heritability (h2snp) was estimated as the proportion of phenotypic variance jointly accounted for by available SNPs in the GWAS studies. LDScore regression (v.1.0.1) (LDSC) was employed to estimate genetic heritability. European LD scores for SNPs were used from the ‘eur_w_ld_chr/’ files7, and the estimates were based on 1,163,161 overlapping SNPs. We also present SNP heritability on the liability scale with a population prevalence of 0.3 for depression-related phenotypes [33].
Polygenic risk scoring
Polygenic risk scoring was used to dissect the genetic relationship between a lifetime anhedonia phenotype and the psychiatric disorders. We selected a range of large-scale GWAS with openly available summary statistics (SS) for psychiatric and somatic phenotypes from the Psychiatric Genomics Consortium (PGC) and UK Biobank (Table S1 in the Supplementary). The selection of psychiatric and somatic phenotypes for the analysis was dictated by the available scientific literature on the association of certain psychiatric disorders and somatic conditions with depression in clinical studies (Table S1 in the Supplementary). The variants with duplicated rsIDs and complementary alleles were discarded. The PRSice-2 software was used to generate the PRS [34]. PRS were investigated for association with a lifetime anhedonia phenotype in the dataset using a logistical regression model including five principal components. We employed the Bonferroni correction of the obtained p-values.
RESULTS
Sample characteristics
The study included 4,520 participants, of whom 50.4% (n=2,280) were female. The mean age of the participants was 36.8 (SD=9.8) years. An episode of anhedonia exceeding 2 weeks during their lifetime was reported by 57.6% (n=2,604) participants, of whom 53.3% (n=1,388) were female. At the time of the study, 11.5% (522) of participants had experienced anhedonia for two consequent weeks (current phenotype).
GWAS analysis
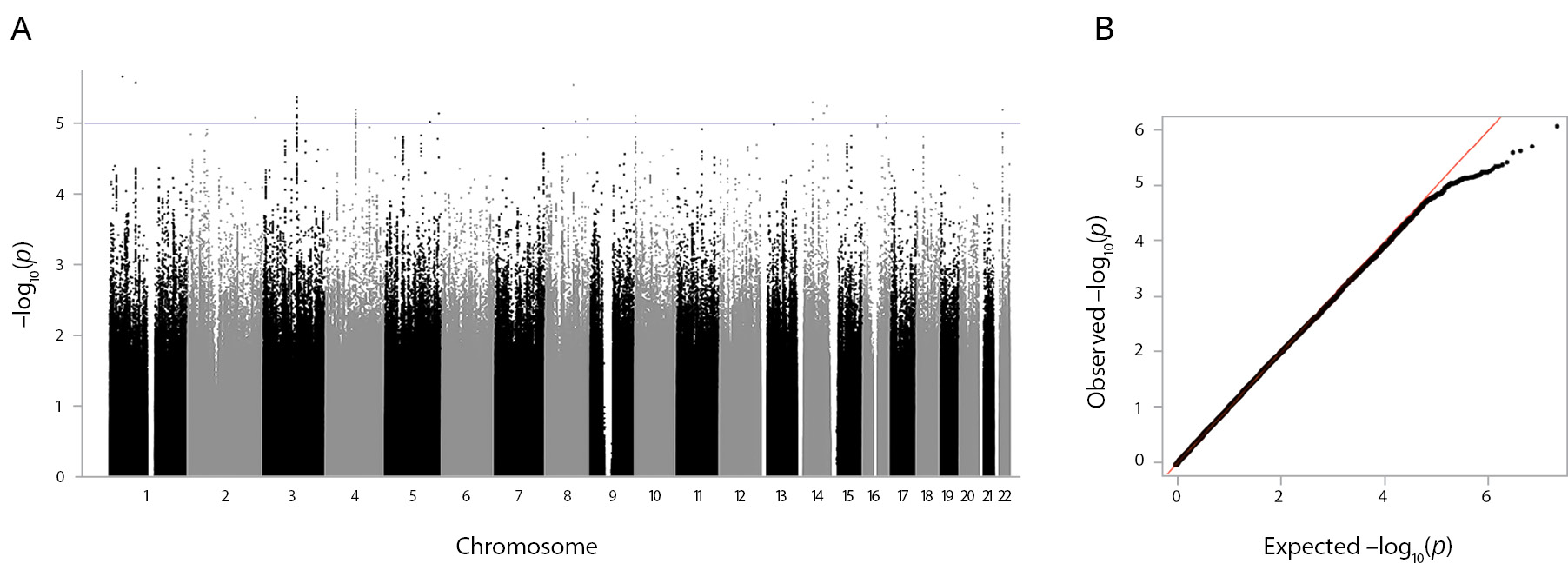
The GWAS on the lifetime anhedonia phenotype did not reveal variants with genome-wide significant association (p <10-8) (Figure 1). The leading five associated variants (p <10-5) are shown in Box S1 in the Supplementary. The most significant (p=9.71×10-7) was the variant rs296009 (chr5:168513184). This SNP is in an intron of the SLIT3 (slit guidance ligand 3) gene, and the risk allele (A) has a frequency of 0.08. The gene list obtained after linking the variants with p <10-5 with likely associated genes using POSTGAP includes 51 genes (Box S1 in the Supplementary). Replication was not performed, because no results with a genome-wide significance level were obtained.
Figure 1. GWAS results of the lifetime anhedonia phenotype. Note: (A) The Manhattan plot for the lifetime anhedonia phenotype. Association analysis p values for each SNP are plotted (as –log10[p]) vs the chromosomal position. The blue line indicates the significance level p <1x10-5. (B) The QQ plot for the lifetime anhedonia phenotype. The QQ plot shows the observed vs expected p-value for every variant.
Enrichment analysis with targets of the ATC drug categories revealed a significant enrichment with B02B (vitamin K and other hemostatics) (p.adj.=0.048, Benjamini-Hochberg correction) and B02 (antihemorrhagics) (p.adj.=0.048, Benjamini-Hochberg correction) (Figure S2, C in the Supplementary). A single gene was driving the enrichment — DUSP1.
SNP-based heritability
SNP-based heritability for the lifetime anhedonia phenotype was h2snp=0.174 (SE=0.09). We also obtained liability-scale heritability considering phenotype prevalence in the population. We used approximated estimations of the prevalence of major depression during the lifetime — 0.3 [33]. Thus, for anhedonia h2snp the liability scale was 0.26 (SE=0.14). These results and their interpretation should be treated with caution due to the small sample size.
PRS analysis
Additional models with the usage of only covariates (age, sex, 15 Multidimensional scalings components for comparative assessment of genetic PRS.R2) and other (Null.R2) factors and complete models, considering both factor groups (Full.R2), were built. The significance threshold with the Bonferroni correction for the psychiatric PRS analysis was 0.05/11=0.0045. As shown in Table S3 (in the Supplementary), PRS for major depression, BD, and schizophrenia were strongly associated with anhedonia, showing that the genetic liability of these disorders increases anhedonia risk. At the same time, PRS for neuroticism and anxiety were not significantly associated with anhedonia (p >0.0045). Nevertheless, nominal significance for neuroticism was noted.
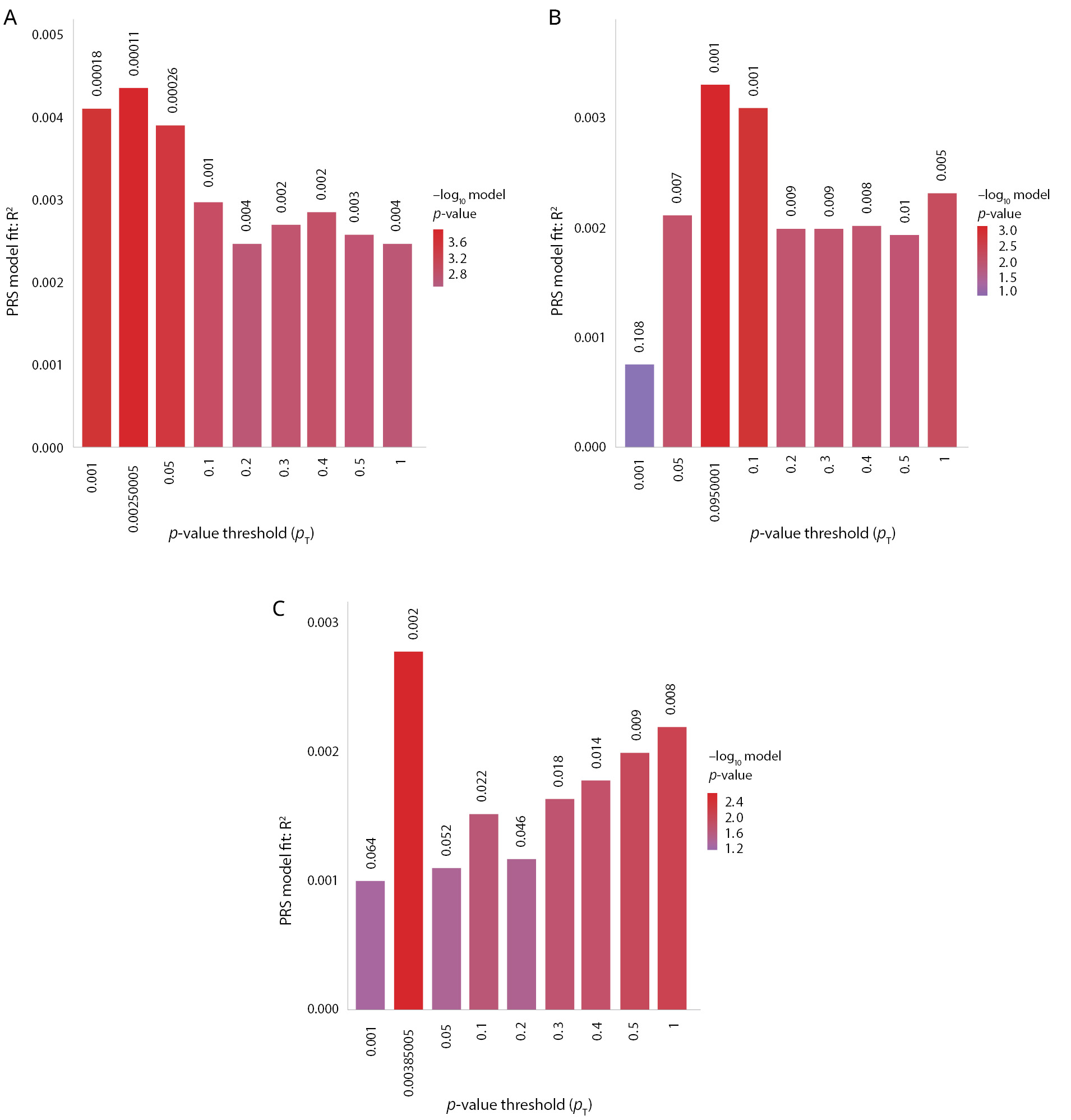
The most significant models of PRSs regarding the prognosis of anhedonia among the three disorders were obtained with the meta-analysis GWAS summary statistics for depression from PGC and UK Biobank (PRS.R2=0.00436498, Full.R2=0.0295311, p=0.00011262), BD from PGC (PRS.R2=0.00329757, Full.R2=0.0284637, p=0.000785365), and schizophrenia from PGC, second wave (PRS.R2=0.00276988, Full.R2=0.02793, p=0.00208176). The quantitative characteristics of the most significant PRSs are shown in Figure 2.
Figure 2. Polygenic risk scores for depression, bipolar disorder, and schizophrenia are significantly associated with the lifetime anhedonia phenotype. Note: The x-axis shows the p-value threshold used to select SNPs from the discovery GWAS: (A) meta-analysis of depression from the PGC and UK Biobank; (B) bipolar disorder PGC; (C) schizophrenia PGC (2nd wave). The y-axis shows the percentage variance explained on the liability scale. p-values of the association between polygenic scores and the lifetime anhedonia phenotype are shown above each bar.
As shown in Table S4 (in the Supplementary), none of the PRS for somatic phenotypes reached the significance level after correction for multiple comparisons (p >0.05/17=0.003). The nominal significance for the association of the lifetime anhedonia phenotype was determined for omega-3 fatty acids, type 2 diabetes mellitus, Crohn’s disease, and ischemic stroke.
Enrichment analyses
Enrichment analysis with the ABA Enrichment package using the set of 51 genes associated with the variants with p <10-5 by POSTGAP shows the highest count of significant enrichments (n=4) in the posterior orbital gyrus (Table S5 in the Supplementary). The region with the smallest minimal family-wise error rate (FWER) with 3 significant enrichments was located in the retrosplenial part of the left cingulate gyrus. Comparison between the expression levels of the genes across the brain regions are shown in Figure S2 (D, E) in the Supplementary. The ATC drug category most significantly enriched in the gene set was B02B — vitamin K and other hemostatics (Table S6, Figure S2 (C) in the Supplementary).
DISCUSSION
This study is the first Russian GWAS of the lifetime anhedonia phenotype based on its DSM-5 criteria of major depression. According to the data of the RDoC transdiagnostic approach, we found that the polygenic component for major depression, BD, and schizophrenia had increased the risks of lifetime anhedonia phenotype. However, we did not find that PRS of somatic conditions could significantly predict the lifetime anhedonia phenotype.
PRS for major depression, BD, and schizophrenia, with the exception of neuroticism and anxiety disorders, were significantly associated with the lifetime anhedonia phenotype. Similar associations were revealed in the largest GWAS of anhedonia of UK Biobank participants [21]. The absence of a genetic link between anhedonia and anxiety disorders aligns well with existing clinical data, where anhedonia is considered a key symptom in the differential diagnosis of major depression and anxiety disorders [16, 35]. However, there is evidence that neuroticism can contribute to anxiety and anhedonia in patients with major depression [36]. The nominal significance for the association of the lifetime anhedonia phenotype was determined for omega-3 fatty acids, type 2 diabetes mellitus, Crohn’s disease, and ischemic stroke, which had been previously confirmed in systematic reviews and meta-analysis of depression [37–40].
Despite the lack of genome-wide significant variants associations with the lifetime anhedonia phenotype in our study, some of the loci identified here include genes with known associations with mood disorders and metabolic phenotypes (Table S2 in the Supplementary). The rs296009 polymorphism of the SLIT3 gene, the most significant SNP in our study, had not been previously reported in the published GWAS. However, other polymorphisms of this gene have been associated with BD (rs7720655) [41], treatment-resistant depression (rs7735612) [42], as well as with cardiometabolic disorders during antidepressant therapy in patients with schizophrenia and BD (rs17665285)[43], leptin level (rs11954861 and rs11954861) [44], height (rs2974438), and body mass index (BMI) (rs76493495) [45, 46]. The rs577951495 polymorphism of the NECAB1 gene had also not been previously detected in published GWAS studies. However, other polymorphisms of this gene were associated with the lifetime smoking index (rs2062882) [47], age of first sexual intercourse (rs3591843) [48], as well as the level of education and Alzheimer’s disease (rs12675931) [49].
High estimates of SNP-based heritability of anhedonia, similar to ours, have been obtained in other studies: 69% [18], 20% [19], 20.4–26.6% [20]. Estimates of SNP-based heritability relate to the data of twin studies in which the heritability level of anhedonia amounted to 44% [50]. At the same time, the lowest SNP-based heritability level (5.6%) was observed in the UK Biobank study with the largest sample size [21]. Such differences can be explained by the characteristics of phenotyping; namely, the use of the lifetime anhedonia phenotype in our study. The bias in the calculation of SNP-based heritability results could also be affected by a small sample size (<5,000).
The set of 51 variants associated with anhedonia with a suggestive threshold (p <10-5) with POSTGAP was significantly overrepresented in the ATC drug category B02B (p.adj.=0.048), which includes vitamin K and other hemostatics, due to DUSP1 — one of the genes the expression of which is affected by vitamin K8. This vitamin has been implicated in the regulation of the sphingolipid metabolism and is protective against oxidative stress in the brain. It has been shown that higher dietary vitamin K intake was significantly associated with a lower level of depressive symptoms, including the fact that individuals with the highest dietary vitamin K intake had lower odds of depressive symptoms (OR=0.58; 95%CI: 0.43–0.80) [51]. Mice with deletion of DUSP1, in turn, are resilient to stress-induced depression [52]. Vitamin K3 decreases the expression of DUSP1, and overexpression of this gene significantly increases cellular susceptibility to oxidative damage [53]. Thus, the antidepressant and anti-oxidative effects of vitamin K could be partially associated with this gene interaction.
Enrichment analysis showed the highest degree of significant enrichment in the posterior orbital gyrus in our study. The posterior orbital gyrus receives inputs from the limbic regions (i.e., amygdala, hippocampus, olfactory cortex, and insula) and plays an important role in processing the olfactory and integration of emotions and memories associated with sensory experiences [54]. According to neuroimaging studies, parts of the orbital gyrus are associated with various manifestations of anhedonia and major depression [21, 55–57].
In summary, ours and other results indicate that anhedonia is a widespread phenomenon in the population, with a complex polygenic architecture that overlaps with a number of phenotypically similar mental disorders and somatic conditions. Moreover, the results of our anhedonia GWAS have significantly enriched our understanding of its biological mechanisms, which for a long time have been associated only with the dopaminergic reward system. Nevertheless, despite repeated attempts at genetically connecting anhedonia with mood disorders and schizophrenia, it remains premature to assert that the mechanisms triggering anhedonia are shared. To demonstrate such patterns, GWAS studies using deep phenotyping of anhedonia are required, considering its clinical characteristics, as well as a subsequent analysis of the biological risk of pathways enrichment. The study of the genetic overlapping of anhedonia and somatic diseases can help in understanding the relationship of these diseases with mental disorders.
Limitations
This study has a range of limitations. The main limitation is its small sample size, which is critical for identifying the variants with genome-wide significance. This could also be the reason for the lack of replication of our GWAS results in an independent sample. The second limitation is the heterogeneity of the anhedonia phenotype considered here: subtypes of anhedonia based on origin (physical/social, consummatory/anticipatory) were not considered. The study sample was assembled on the basis of the clients of a private genetic testing company, which could affect the socio-demographic characteristics of the participants as compared to the general population. Nevertheless, we believe that our results are relevant for a wide range of future studies, including replication analyses for GWAS on a wide range of psychiatric conditions, of which anhedonia is one.
CONCLUSION
Anhedonia has a complex polygenic architecture that overlaps with a number of other phenotypically similar psychiatric disorders and somatic conditions. This study demonstrates that genetic liability for schizophrenia, BD, and major depression increases the risk of a lifetime anhedonia phenotype. At the same time, we did not uncover common genetic factors between anxiety and anhedonia, which aligns well with existing clinical evidence. In addition, none of the PRS for somatic phenotypes reached the significance level after correction for multiple comparisons. Thus, the best predictive models were based on summary statistics of mental disorders. This fact may indicate that the appearance of anhedonia in somatic disorders or normal conditions may develop due to a genetic predisposition to mood disorders or schizophrenia. Further collaborative efforts to study the transdiagnostic nature of anhedonia would make it possible to identify reliable genetic associations and improve our understanding of the etiology of anhedonia.