INTRODUCTION
The role of inflammation and immune system disorders in the pathogenesis of depression has been studied for several decades [1, 2]. As a result, numerous clinical findings have confirmed the connection between neuroinflammation and the development of depression [3, 4]. Additionally, patients with depression have been found to have high concentrations of interleukin-6 (IL) and the C-reactive protein (CRP) [5, 6], to show signs of microglial activation [7] and increased levels of kynurenine associated with the effect of the neuroinflammation on serotonin synthesis [4]. It has also been shown that the pro-inflammatory cytokine interferon (IFN) α induces depression as a side effect [8, 9]. Moreover, patients who received higher doses of IFNα for 24 weeks showed more severe depressive symptoms [9]. In contrast, it appears that some drugs with anti-inflammatory activity may also have antidepressant effects [4]. The antidepressant effect has also been studied in nonsteroidal anti-inflammatory drugs, cytokine inhibitors, statins, polyunsaturated fatty acids, and corticosteroids, but the results have been contradictory [10].
Some authors critically evaluate the formal diagnostic approach (based on Diagnostic and Statistical Manual of Mental Disorders, DSM and the International Classification of Diseases, ICD) to the verification of depression in major depressive disorder (MDD) and bipolar disorder (BD), considering their pathogenetic and clinical features [11]. In patients with BD, the final diagnosis is usually made 6–8 years after the first affective phases [12]. The most common incorrect diagnosis is MDD, which is related to the typical onset of BD with depressive phases and the delayed onset of hypo/manic phases or difficulties in retrospectively verifying them [13]. As a result, antidepressants are often mistakenly prescribed for the treatment of the current depressive episode, leading to pharmacogenetic phase inversions and the development of mixed episodes and rapid cycling in patients with BD [14].
It seems logical to consider the differences in the pathogenesis of depression in MDD and BD [15, 16]. In this regard, molecular, genetic, and neuroimaging studies of specific markers that can differentiate depressive episodes in BD and MDD on a neurobiological basis are highly relevant [17]. Studies of immunological markers in peripheral blood in BD and MDD have revealed disruptions in immune response regulation [18, 19]. It is suggested that these markers could be used to improve the accuracy of the differential diagnosis of BD and MDD [20].
However, there is a lack of studies on the specific immune system and systemic inflammation markers that compare current depression in BD and MDD, especially in conjunction with clinical manifestations and disorder course features. These findings are crucial for further understanding whether both phenotypes are biologically continuous conditions within the same spectrum of pathophysiological changes, or whether BD and MDD are independent conditions with different pathophysiological bases [21–23]. Comparison of immune system and systemic inflammation markers in depression in patients with MDD and BD is necessary for identifying the biomarkers of these disorders, finding new psychopharmacological targets, and predicting the effectiveness of the standard treatment for BD and MDD.
To our knowledge, scoping reviews, systematic reviews, and meta-analyses comparing immune system and systemic inflammation markers in a current depressive episode in patients with BD and MDD have not been conducted. On this basis, we conducted a systematic literature analysis using the scoping review methodology to describe and summarize the results of these studies.
METHODS
The description of the review was carried out in accordance with the recommendations (checklist of control questions) from the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines, modified for scoping reviews [24]. The protocol for this study has not been registered in public sources. The protocol can be obtained by sending a justified request to the corresponding author.
Eligibility criteria
Inclusion criteria:
The review included articles published in peer-reviewed journals in English or Russian, containing the results of studies on patient groups with current depressive episodes in MDD and BD (type I or II), comparing immune system and systemic inflammation markers (immunoglobulins (Ig) A, M, G, and E, autoantibodies, cytokines, complement components, acute-phase proteins, growth factors, erythrocyte sedimentation rate, blood cell counts, ratios, and functions). Diagnosis of MDD and BD was based on the criteria of the DSM-IV, DSM-5 or the ICD-10.
Exclusion criteria:
- Case report studies.
- Full or partial data duplication (in the case of partial duplication, the publications with the largest sample size were included in the review).
- Absence of mean or median values for immune system and systemic inflammation markers and/or results of statistical comparisons between the patient groups with BD and MDD.
- Studies that have analyzed mixed patient groups (including patients with dysthymia, cyclothymia, schizophrenia spectrum disorders).
- The article with the research results is behind a paywall or the author did not grant access upon request.
Information sources
The search was conducted in the electronic databases Medline and eLIBRARY. The search period covered ran from January 1994 to December 2022. The search was limited to 1994, because that year marked the release of DSM-IV, which contained the first description of the diagnostic criteria for BD II. The search was carried out in December 2023.
Search strategy
The search query in the Medline database included the following combination of keywords and search operators: [(unipolar depression) OR (major depressive disorder) OR (recurrent depressive disorder)] AND [(bipolar depression) OR (bipolar disorder) OR (bipolar affective disorder) OR (bipolar disorder I type) OR (bipolar disorder II type)] AND [(immunological alterations) OR (immunomarkers) OR (immunological markers) OR (immunological) OR (immune-inflammatory profiles) OR (cytokines) OR (immunity) OR (neuroinflammation) OR (inflammation) OR (immune system)]. The search query in Russian in the eLIBRARY database included: [(unipolar depression) OR (major depressive disorder) OR (recurrent depressive disorder)] AND [(bipolar depression) OR (bipolar disorder) OR (bipolar affective disorder) OR (bipolar I disorder) OR (bipolar II disorder)] AND [(immunological alterations) OR (immunomarkers) OR (immunological markers) OR (immunological) OR (immune-inflammatory profiles) OR (cytokines) OR (immunity) OR (neuroinflammation) OR (inflammation) OR (immune system)]. The search query was formulated by (AK) and approved by all co-authors. When searching the Medline electronic database, additional time filters, as mentioned above, were used. When searching in the eLIBRARY database, the following filters were applied: search by title and abstract; publication type: journal article.
Selection process
The primary screening of potentially relevant articles was conducted by reviewing their titles and abstracts and performing a preliminary assessment if they meet the eligibility criteria. The selected articles were listed for further review of their full texts and selection of relevant studies that met all the planned inclusion and exclusion criteria. The screening and content review of the articles were performed (AK) and confirmed by two other authors of the review (PS, NP). The final decision in case of disagreements regarding the included articles was made by one author (NP).
Data extraction
A standardized form in the format of an electronic spreadsheet was used for data extraction. The following data were extracted from the relevant (selection criteria-compliant) articles: title, authors, year of publication, country, study design, patient sample size, biological material, study method, immune system and systemic inflammation markers, drug therapy at the time of participation in the study, and group comparison results considering sex or type of BD. Data were extracted by one author (AK) and then double-checked and confirmed by the other authors of the review (PS, OL, and NP). Disagreements were resolved by one author (NP).
Critical assessment
No critical assessment of the sources included in the review was conducted.
Analysis of results
A descriptive analysis of the selected sources was performed. The results of the comparison of the BD and MDD groups based on immune system markers and systemic inflammation values in each study were described using categories such as “Higher in BD” and “Higher in MDD” if differences were statistically confirmed, and “No differences” if no significant differences were found. We also analyzed the relationship between immune system markers, systemic inflammation, and various clinical characteristics of depressive episodes in BD and MDD found in the included studies.
RESULTS
Source selection
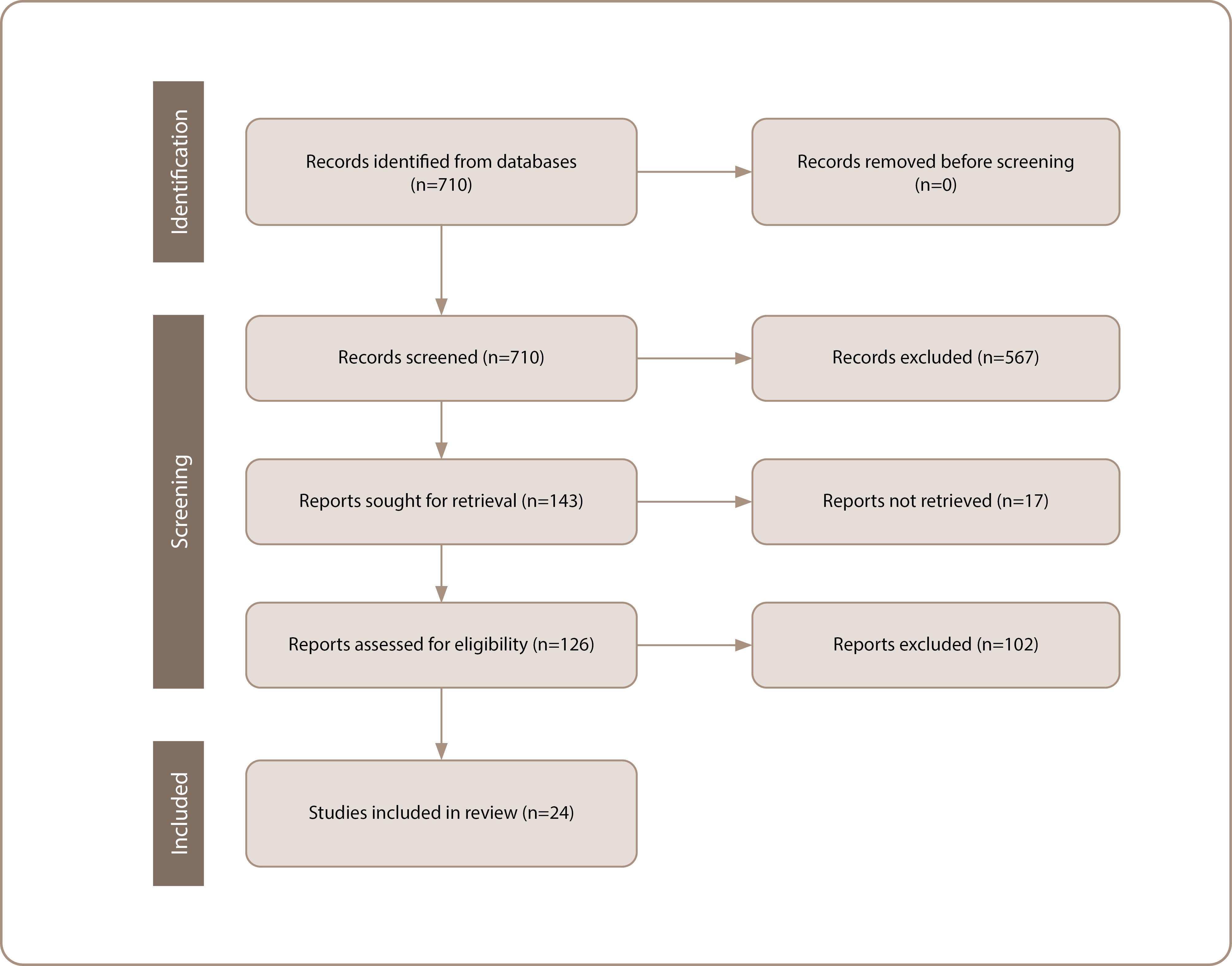
As a result of the database search, 710 articles were netted, all in Medline. After reviewing the titles and abstracts, 143 articles were deemed potentially relevant, of which 126 were publicly available or provided by the authors. After reading the full texts of the articles, 24 original studies that met the eligibility criteria were included in the final analysis (Figure 1) [18, 20, 25–46].
Figure 1. PRISMA flow chart of the literature search and the selection process. Source: Kasyanova et al., 2024.
Characteristics of the sources
A total of 2,785 patients with BD and 10,944 patients with MDD participated in 24 studies included in the review. The studies were published between 2007 and 2022. Of the 24 studies, 20 used a cross-sectional design, while the remaining four employed different approaches: cohort studies (n=2), case-control studies (n=1), and a double-blind placebo-controlled study (n=1). Most studies were conducted by authors from Europe (Italy — four studies, Bulgaria — three, Belgium — two, Turkey — two; Romania, Poland, Germany, Netherlands — one each), Asia (China — five studies; Taiwan — four; Thailand — three), and North America (Canada and the USA — four studies each). Additionally, three studies were conducted by authors from Australia, and one from Brazil. No studies in Russian that met the inclusion criteria were identified.
Results of studies
Table 1 presents the results of the comparison of immune system and systemic inflammation markers during a current depressive episode in patients with BD and MDD. Promising markers of the current depressive episode in BD include chemokines (C-C motif chemokine ligand 3 (CCL3), CCL4, CCL5, CCL11), platelet-derived growth factor B (PDGFB), and IL-9 (with two confirming studies for each). In MDD, promising markers include soluble tumor necrosis factor receptor 1 (sTNFR1) and IgG to oxidized low-density lipoproteins (LDL) (with two studies for each). Patients with BD and MDD had comparable concentrations of IL-8 (reported in five studies), IL-2 and IL-10 (in 4 studies), IL-13 and IFN-γ (in three studies); IL-17, IL-1Rα, vascular endothelial growth factor, leukocyte, monocyte, and platelet counts (in two studies). Contradictory results were obtained for TNF-α (no differences found in five studies, increased level in BD in five, increased level in MDD in two), IL-6 (no differences found in 8 studies, increased level in BD in four), CRP (no differences found in six studies, increased level in BD in two), IL-4 (no differences found in three studies, increased level in MDD in two), IL-1β, and neutrophils (no differences found in one study, increased level in BD in two).
Table 1. Study of immune system and systemic inflammation markers during depressive episode in patients with bipolar disorder and major depressive disorder
|
Source |
Country |
Study design |
Sample size, abs. |
Biomaterial (study method) |
Therapy* |
Indicators higher in BD |
Indicators higher in MDD |
No differences (BD vs MDD) |
|
|
BD |
MDD |
||||||||
|
Comai et al., 2022 [25] |
Italy, Canada |
Сross-sectional** |
66 |
100 |
Plasma (multiplex assay) |
Yes |
IL-1β, IL-2, IL-6, IL-9, CCL11, CCL3, PDGF-B, CCL4, CCL5, TNF-α |
IL-4, IL-7 |
IL-1Rα, IL-8, IL-10, IL-12, IL-13, IL-15, IL-17, IFN-γ, MCP-1 (CCL2), CXCL10, FGF, G-CSF, GM-CSF, VEGF |
|
Wei et al., 2022 [26] |
China |
Сross-sectional |
1,664 |
8,899 |
Whole blood (blood chemistry) |
No data |
MPV, neutrophils, lymphocytes |
Platelets, RDW, PLR, PAR |
PDW, PCT, RPR |
|
Bulut et al., 2021 [27] |
Turkey |
Сross-sectional** |
70 |
93 |
Whole blood (blood chemistry) |
No data |
Not detected |
Not detected |
NLR, PLR |
|
Caldirola et al., 2021 [28] |
Italy |
Сross-sectional |
135 |
156 |
Serum (ELISA) |
Yes |
CRP (> 3 and ≤ 10 mg/L) |
Not detected |
Not determined |
|
Dionisie et al., 2021 [29] |
Romania |
Сross-sectional |
34 |
83 |
Whole blood (blood chemistry, complete blood count) |
Yes |
Neutrophils, NLR, SII |
Lymphocytes |
White blood cells, monocytes, platelets, PLR, MLR |
|
Huang et al., 2021 [30] |
Taiwan |
Сross-sectional** |
33 |
66 |
Serum (ELISA) |
Yes |
TNF-α, IL-6 |
Not detected |
CRP, IL-2, MCP-1 (CCL2), P-selectin |
|
Karadağ et al., 2021 [31] |
Turkey |
Сross-sectional |
31 |
25 |
Serum (ELISA) |
Yes |
Not detected |
Not detected |
TRAIL, TWEAK, CRP |
|
Poletti et al., 2021 [32] |
Italy |
Сross-sectional** |
81 |
127 |
Plasma (multiplex assay) |
Yes |
IL-1β, IL-9, IL-16, TNF-α, MIF, CCL1, MCP-1 (CCL2), CCL3, CCL4, CCL5, CCL8, CCL11, CCL13, CCL21, CCL22, CCL25, CCL26, CCL27, CXCL1, CXCL6 , CXCL9, CXCL10, CXCL11, СXCL16, CX3CL1, bFGF, PDGF-B |
Not detected |
IL-1Rα, IL-2, IL-4, IL-6, IL-7, IL-8, IL-10, IL-13, IL-17, CCL7, CCL15, CCL17, CCL19, CCL20, CCL23, CCL24 , CXCL2, CXCL5, CXCL8, CXCL12, CXCL13, VEGF |
|
Brunoni et al., 2020 [18] |
Brazil, USA, Thailand, Australia, Canada, Bulgaria |
Сross-sectional |
59 |
245 |
Plasma (ELISA, flow cytometry) |
Patients with MDD – no, with BD – yes |
IL-6, sTNFR2, IL-18, IL-33, sST2, klotho |
IL-1β, TNF-α, sTNFR1, IL-12, IL-10 |
IL-8, IL-12p70 |
|
Simeonova et al., 2020, a [33] |
Bulgaria, Belgium, Canada, Poland, Thailand, Australia |
Сross-sectional** |
66 |
44 |
Serum (ELISA, colorimetric assay) |
Yes |
IgA to LPS of Pseudomonas putida and Citrobacter koseri |
IgG to oxidized LDL, IgM to LPS of Hafnia alvei (in comparison with BD II) |
Total peroxides, IgM to LPS of Morganella morganii, Pseudomonas aeruginosa, P. putida, C. koseri, Klebsiella pneumoniae, IgA to LPS of H. alvei, P. aeruginosa, M. morganii, K. pneumoniae, total IgA/IgM to LPS of gram-negative bacteria |
|
Simeonova et al., 2020, b [34] |
Bulgaria, Belgium, Thailand, Australia |
Сross-sectional** |
54 |
47 |
Serum (ELISA, colorimetric assay) |
Yes |
Not detected |
IgM to MDA, oleic acid, Pi, total IgM to OSEs, IgG to oxidized LDL (compared to BAR II); total peroxides (compared to BAR I) |
IgM to LPS of H. alvei, P. aeruginosa, M. morganii, P. putida, C. koseri, K. pneumoniae, IgA to LPS of H. alvei, P. aeruginosa, M. morganii, P. putida, C. koseri, K. pneumoniae, IgM to azelaic acid, total IgM to NO adducts |
|
Lu et al., 2019 [35] |
China |
Case control |
26 |
21 |
Serum (ELISA, immunoturbidimetric assay) |
No |
Not detected |
Not detected |
IL-6, IL-8, CRP |
|
Mazza et al., 2019 [36] |
Italy |
Сross-sectional |
40 |
36 |
Whole blood (blood chemistry) |
No data |
Not detected |
Not detected |
Leukocytes, neutrophils, lymphocytes, monocytes, platelets, NLR, MLR, PLR |
|
Mao et al., 2018 [20] |
China |
Cohort** |
61 |
64 |
Plasma (multiplex assay) |
Yes |
TNF-α, IL-4 (after 12 weeks of treatment) |
TNF-α and IL-13 before treatment, TNF-α and IL-4 after 12 weeks of treatment in patients who responded to treatment |
IL-4, IL-6 (before treatment). IL-13, IL-6 (after 12 weeks of treatment in patients who responded to treatment) |
|
Chang et al., 2017 [37] |
Taiwan |
Cohort** |
88 |
72 |
Plasma (ELISA) |
No |
CRP |
Not detected |
Not detected |
|
Hage et al., 2017 [38] |
USA |
Сross-sectional** |
37 |
64 |
Plasma (ELISA) |
Patients with MDD – no, with BD – yes |
ИЛ-10, MCP-1 (CCL2) |
CRP |
TNF-α, IL-6, IL-8, IL-1β |
|
Park et al., 2017 [39] |
USA |
Double-blind, placebo-controlled study |
31 |
49 |
Plasma (multiplex assay) |
Yes |
TNF-α, IL-6, IL-8 |
sTNFR1 (higher at baseline, 230 min, and 1 day, but not 3 days after ketamine injection) |
IFN-γ, IL-2, IL-5, IL-10 |
|
Ren et al., 2017 [40] |
China |
Сross-sectional** |
30 |
30 |
Plasma (liquid chromatography with tandem mass spectrometry) |
No |
Alpha-1-acid glycoprotein, C-mannose receptor type 2, antileukoproteinase |
Serotransferrin, pantetheinase, apolipoprotein A-I, endoglin, suprabasin, sulfhydryl oxidase |
Not detected |
|
Wu et al., 2017 [41] |
China |
Сross-sectional** |
23 |
22 |
Whole blood, plasma (flow cytometry) |
No |
Not detected |
CD3+CD8+ cytotoxic T cells |
CD3+ T cells, CD3+CD4+ Th cells, CD3-CD16+CD5+ NK cells, TIM-3, PD-1, PD-L1, PD-L2, IFN-γ, TNF-α, IL-2, IL-4, IL-10, IL-6 |
|
Schaefer et al., 2016 [42] |
Germany |
Сross-sectional** |
22 |
11 |
Serum (ELISA) |
Yes |
Not detected |
Not detected |
sICAM-1 |
|
Becking et al., 2015 [43] |
Netherlands |
Сross-sectional** |
124 |
640 |
Plasma (ELISA) |
No data |
Not detected |
Not detected |
TNF-α, IL-6, CRP |
|
Manalai et al., 2012 [44] |
USA |
Сross-sectional** |
39 |
55 |
Serum (immunofluorescence assay) |
Yes |
Not detected |
Not detected |
IgE to tree and ragweed allergens (seropositive/seronegative status) |
|
Su C et al., 2011 [45] |
Taiwan |
Сross-sectional** |
10 |
18 |
Serum (ELISA), plasma (immunoturbidimetric assay) |
No |
Not detected |
Not detected |
CRP, TNF-α, IL-6 |
|
Hung et al., 2007 [46] |
Taiwan |
Сross-sectional** |
15 |
21 |
Serum (ELISA), plasma (immunoturbidimetric assay) |
No |
Not detected |
Not detected |
CRP, TNF-α, IL-6 |
Note: * Participant’s treatment at study entry; ** Design not specified in cited papers, determined by the authors of this review. bFGF — basic fibroblast growth factor; CCL11 — C-C motif chemokine ligand 11; CXCL10 — C-X-C motif chemokine ligand 10; FGF — fibroblast growth factor; IgA — immunoglobulin A; IgE — immunoglobulin E; IgG — immunoglobulin G; IgM — immunoglobulin M; MCP-1 — monocyte chemoattractant protein 1; MDA — malondialdehyde; MIF — macrophage migration inhibitory factor; MLR — monocyte-to-lymphocyte ratio; MPV — mean platelet volume; NLR — neutrophil-to-lymphocyte ratio; NK cells — natural killer cells; NO — nitric oxide; OSE — oxidation-specific epitopes; PAR — platelet-to-albumin ratio; PCT — plateletcrit; PD-1 — programmed cell death protein 1; PDGF-B — platelet-derived growth factor B; PD-L1 — programmed death-ligand 1; PDW — platelet distribution width; Pi — phosphatidylinositol; PLR — platelet-to-lymphocyte ratio; RDW — red cell distribution width; RPR — red cell distribution width-to-platelet ratio; sICAM-1 — soluble intercellular adhesion molecule-1; SII — systemic immune-inflammation index; sST2 — soluble suppression of tumorigenicity 2; sTNFR2 — soluble tumor necrosis factor receptor 2; Th cells — T-helper lymphocytes; TIM-3 — T-cell immunoglobulin and mucin-domain containing-3, immunoglobulin and mucin/mucin domain; TRAIL — TNF-related apoptosis-inducing ligand; TWEAK — TNF-like weak inducer of apoptosis; VEGF — vascular endothelial growth factor; BD — bipolar disorder; G-CSF — granulocyte colony-stimulating factor; GM-CSF — granulocyte-macrophage colony-stimulating factor; IL-1β — interleukin-1beta; IFN-γ — interferon gamma; ELISA — enzyme-linked immunosorbent assay; LDL — lipoproteins; LPS — lipopolysaccharide; MDD — major depressive disorder; CRP — C-reactive protein; TNF-α — tumor necrosis factor-α.
The type of BD was considered in three studies when analyzing immune system and systemic inflammation markers. In the study by Brunoni et al. [18], no differences in the studied markers (IL-6, TNF-α, sTNFR2, etc.) were found between patients with BD I and II. The study by Simeonova et al. [33] found that in BD I, compared to BD II, the total concentrations of IgM to NO adducts, lipopolysaccharide (LPS) of Morganella morganii and Citrobacter koseri, IgA to LPS of Pseudomonas putida and Citrobacter koseri, and the integral index ratio of IgM/IgA to LPS of all Gram-negative bacteria were higher. Higher concentrations of IgM and IgA to LPS of Gram-negative bacteria in BD I were also found in a third study [34].
The biological sex of patients was also considered in three studies when analyzing immune system and systemic inflammation markers. The study by Wei et al. [26] showed that in patients with MDD, neutrophil and lymphocyte counts were higher in men compared to women. In patients with BD, neutrophil and lymphocyte counts in men were also higher, while platelet counts were lower than in women [26]. In the study by Lu et al. [35], it was found that in patients with MDD and BD during depressive episodes, the concentration of IL-8 in serum was higher in men than in women. In the study by Becking et al. [43], no statistically significant differences were found in the concentrations of TNF-α, IL-6, and CRP when comparing men with MDD and BD, as well as separately comparing women with MDD and BD. Comparison of immune system and systemic inflammation marker values between men and women was not performed in this study.
In patients with MDD and BD, positive correlations were found between various immune system and systemic inflammation markers and the severity of depressive and anxiety symptoms, the melancholic subtype of depression, age of onset of mood disorder, and the body mass index (Table 2). Negative correlations were also observed between various immune system and systemic inflammation markers and the imipramine equivalent and the level of anxiety symptoms in both patient groups.
Table 2. The relationship between immune system and systemic inflammation markers and the clinical characteristics of depression in bipolar disorder and major depressive disorder
|
Clinical characteristic |
Immune system and systemic inflammation markers |
|
|
BD |
MDD |
|
|
Severity of depressive symptoms (positive relationship) |
CRP [37], sTNFR1 [39], IgA to LPS of Pseudomonas putida, Citrobacter koseri, Hafnia alvei, Pseudomonas aeruginosa, Morganella morganii, Klebsiella pneumoniae [33], IgM to azelaic acid and oleic acids, MDA and Pi, total IgM to OSE [34], + CCL4 [25] in patients with MDD |
– |
|
Level of anxiety (positive relationship) |
sTNFR1, sTNFR1/sTNFR2 ratio [18] |
– |
|
Level of anxiety (negative relationship) |
sTNFR2 [18] |
– |
|
Severity of hypo/manic symptoms (positive relationship) |
CRP [35] |
Not determined |
|
Age of disorder onset (negative relationship) |
CD3+CD8+ cytotoxic T lymphocytes [41] |
Not determined |
|
Melancholic type of depression (positive association) |
IgA to LPS of C. koseri [33] |
|
|
Anxiety as a personality trait (positive relationship) |
sTNFR1, sTNFR1/sTNFR2 ratio [18] |
– |
|
Body mass index (positive relationship) |
– |
|
|
Imipramine equivalents (negative relationship) |
– |
|
Note: CCL11 — C-C motif chemokine ligand 11; IgA — immunoglobulin A; IgM — immunoglobulin M; MDA — malondialdehyde; OSE — oxidation-specific epitopes; Pi — phosphatidylinositol; sTNFR1 — soluble tumor necrosis factor receptor 1; TIM-3 — T-cell immunoglobulin and mucin-domain containing-3. BD — bipolar disorder; IL-1β — interleukin-1beta; LPS — lipopolysaccharide; MDD — major depressive disorder; CRP — C-reactive protein; TNF-α — tumor necrosis factor-α.
DISCUSSION
Key findings
For the first time, the results of original studies investigating immune system and systemic inflammation markers in current depression among patients with MDD and BD were summarized. The data related to the most commonly investigated immune system and systemic inflammation markers (IL-1β, IL-4, IL-6, TNF-α, CRP, neutrophil count) are contradictory. Differences in the concentrations of IL-2, IL-8, IL-10, IL-13, IFN-γ, IL-17, IL-1Rα, vascular endothelial growth factor, leukocyte, monocyte, and platelet counts in patients with BD or MDD were not identified in any of the studies. However, promising biomarkers for the differential diagnosis of a current depressive episode were shown to include chemokines (CCL3, CCL4, CCL5, CCL1), PDGFB, IL-9 (higher concentrations in BD), as well as sTNFR1 and IgG to LDL (higher concentrations in MDD). Several studies have identified associations between immune system and systemic inflammation markers and the clinical characteristics of the mood disorders under investigation.
Limitations
The present review has several important limitations. First, the broad inclusion criteria meant that the studies reviewed had examined different components of immunity. Consequently, despite the number of included studies and the final sample size, some potentially interesting immune and systemic inflammation markers had been investigated in only a small number of studies. Second, although all the studies had excluded conditions that could influence immune and systemic inflammation marker levels, it is impossible to completely rule out this and other confounding factors because the included studies varied in how the authors accounted for comorbid conditions or excluded them (e.g., through clinical examination, patient self-reporting, or medical records). Third, in 14 (58%) of the 24 studies, immune system and systemic inflammation markers were assessed during ongoing pharmacotherapy for mental disorders, which could have affected the results [47]. Fourth, 17 out of 143 potentially relevant studies were unavailable as full-text articles and were therefore excluded from the review. As a result, potentially significant findings regarding the relationship between immune and systemic inflammation markers and depressive episodes in MDD or BD patients might have been overlooked. The original article search was conducted by a single author without reviewing the reference lists of the included papers, increasing the risk of missing relevant studies. Most of the studies included in this review were cross-sectional, making it impossible to infer causal relationships between immune and systemic inflammation markers and depressive episodes in MDD or BD patients.
Comparisons with existing literature
The numerous conflicting findings regarding the association of immune system and systemic inflammation markers with current depressive episodes in MDD and BD can be attributed to the challenges in the psychiatric classification of these disorders. Until 1980, both conditions were grouped under the manic-depressive disorders category, but some researchers now view them as two extremes of a bipolar spectrum [48, 49]. Given this, it is logical that MDD and BD share more similarities than differences. Nevertheless, even subtle distinctions can be crucial in terms of their differential diagnosis. Below, we present the available data on the immune system and systemic inflammation markers identified in this review as the most promising for distinguishing between major depressive disorder and bipolar disorder.
In relation to BD, changes in the concentrations of various cytokines have been the primary focus of previous research. However, the association of chemokines with this disorder has also been confirmed by the systematic reviews conducted by Stuart et al. [50] and Misiak et al. [51]. Chemokines are a family of cytokines capable of inducing directed migration (chemotaxis), particularly to sites of inflammation. All chemokines interact with transmembrane receptors linked to G-proteins, which are expressed, among other locations, within the vascular network of the blood-brain barrier [52]. The link between chemokines and mental disorders is mediated by their neuromodulatory and neurotransmitter-like effects, as well as their role in regulating neurogenesis [50, 51]. The effects of chemokines on the brain are complex, as these proteins exhibit both neuroprotective and neurotoxic properties [53]. Understanding the role of chemokines in the pathogenesis of BD may open new prospects for the development of therapeutic strategies aimed at modulating inflammatory processes and immune regulation.
PDGFB, a growth factor stored in platelet granules and released upon activation, plays a role in blood vessel formation, proliferation, and the migration of mesenchymal cells [54]. PDGF-mediated signaling has been shown to regulate various brain functions, including neurogenesis [55, 56]. Additionally, PDGF-BB inhibits the N-methyl-D-aspartate receptor (NMDAR)-mediated component of excitatory synaptic currents [57, 58], while glutamatergic neurotransmission via NMDAR is implicated in the pathophysiology of MDD and BD [58, 59]. Idemoto et al. [58] suggested that PDGF-BB might contribute to NMDAR dysfunction in mood disorders and mediate pathophysiological differences between MDD and BD. Serum PDGF-BB levels were found to be significantly lower in MDD groups during both depressive phase and euthymic state compared to BD groups and healthy controls. These findings suggest that serum PDGF-BB could be a potential biomarker for the differential diagnosis of BD and MDD. The authors hypothesize that decreased PDGF-BB levels in MDD may be linked to diminished neuroprotective activity and the activation of NMDAR-mediated excitotoxicity in the brain [58]. Several studies have also identified a relationship between PDGF-BB levels and the microstructure of white matter in depressive patients [25, 60, 61]. Moreover, Benedetti et al. [60] demonstrated an inverse relationship between cytokine and PDGF-BB levels and the integrity of myelin sheaths during demyelination [60]. White matter structural abnormalities are known to be associated with the risk of mood disorders, adverse childhood experiences, affective phases, and cognitive impairments in BD [25, 62, 63], as well as the duration of the disorder, treatment resistance, and depression severity in MDD [25, 64, 65].
IL-9 is a pleiotropic cytokine primarily produced by type 2 T-helper cells (Th2), as well as by Th17 cells, regulatory T cells (Treg), and a Th9 subpopulation [54]. This cytokine promotes survival and activates mast cells, epithelial cells, B cells, and T cells [54]. IL-9 may contribute to inflammatory diseases and serves as a key molecule in the differentiation of Th17 and regulation of Treg function [66]. The role of IL-9 in depression may involve its influence on glutamatergic transmission. Th17 cells, through IL-17, reduce the expression of glutamate transporters [67]. A recent study by Poletti et al. showed a positive correlation between IL-9 and glutamate concentrations in the brains of BD patients [53]. The same study also found that IL-1β and CCL5 were associated with higher concentrations of myo-inositol and N-acetylaspartate in the anterior cingulate cortex, respectively [53]. Thus, the impact of cytokines, including IL-1β and IL-9, on the pathogenesis of BD may be mediated through their involvement in neuroinflammatory processes, effects on neuroplasticity and brain metabolism, and their roles in neurotransmission and neurodegeneration.
High concentrations of TNF-α in patients with MDD have been confirmed by several meta-analyses [68, 69, 70] and an umbrella review [71]. TNF-α, a molecule of the innate immune system, is primarily produced by macrophages, natural killer cells, and T lymphocytes [54]. TNF exists in both transmembrane and soluble forms, functioning through binding to tumor necrosis factor receptors 1 and 2 (TNFR1 and TNFR2) [72]. TNFR1 is expressed in all human tissues, while TNFR2 is predominantly expressed in immune and endothelial cells, as well as neurons [73]. Both transmembrane and soluble TNF activate TNFR1, leading to proteolysis of the extracellular domain of TNFR1, which is then released into the bloodstream as sTNFR1 [72, 74]. The production and activity of TNF-α are regulated through the control of TNF gene expression and the release of TNF and its receptors in response to various agonists [75]. It has been suggested that soluble cytokine receptors may better reflect cytokine activity, as they have a longer half-life, are more stable in measurements, and can be detected in plasma even when cytokine levels are undetectable [76, 77]. Thus, sTNFR1 may serve as a marker of systemic inflammation intensity [78, 79]. Peripheral concentrations of sTNFR1 and sTNFR2 have been studied in various mental disorders, but the role of sTNFR1 in the development of MDD remains unclear. Neuronal TNFR1 is thought to contribute to neuroinflammation and demyelination, as well as axonal damage, oligodendrocyte loss, and the induction of glial cell autophagy through the enhancement of oxidative stress in neurons [80].
Increased concentrations of IgG targeting oxidized LDL in MDD indicate autoimmune responses associated with systemic inflammation — a known mechanism in the pathogenesis of depression [33]. These autoimmune IgG responses in MDD patients are linked to dysregulated lipid-targeting antioxidant defense and repair mechanisms [34]. This is driven by oxidative stress and chronic inflammation [34]. This relationship is particularly noteworthy, because concentrations of IgG to oxidized LDL are directly associated with a coronary artery disorder, which often co-occurs with both MDD and BD [33, 81].
Prospects for future research
Further research into immune system and systemic inflammation markers for distinguishing between MDD and BD offers promising directions for the scientific community. These directions may include:
- The transition from studying individual immune and inflammatory markers to a comprehensive immunoprofiling to determine complex changes in concentrations of cytokines (including chemokines), immunoglobulins, lymphocyte, subpopulations, and other markers in peripheral blood or other biological media. Immunoprofiling provides a broad view of the immune response and can help identify the specific immune profiles associated with MDD and BD.
- For a better understanding of the dynamics of immune and inflammatory markers and their associations with the pathogenesis, progression, and prognosis of MDD and BD, prospective studies are needed. Such studies will allow one to track changes in the immune status depending on the disorder phase and therapy effectiveness.
- Studying disorders beyond the unipolar-bipolar dichotomy: Considering that numerous immune and inflammatory markers are similarly elevated in depressive episodes in both MDD and BD, future research should focus on specific clinical features, such as early onset, high recurrence of affective episodes, subthreshold hypomanic and mixed symptoms, family history, and treatment response.
- Understanding the role of inflammatory and immune markers in the pathogenesis of MDD and BD is essential for developing novel therapeutic strategies aimed at modulating the immune system. For example, drugs targeting specific chemokines or their receptors could represent a promising direction in mood disorder treatment.
- The progress in understanding the role of the immune system in mood disorders requires close interdisciplinary cooperation among psychiatrists, immunologists, and neurobiologists. Such collaboration is crucial for integrating data from diverse fields to create a comprehensive view of the pathogenesis of MDD and BD.
CONCLUSION
The results of this scoping review highlight the complexity and multifaceted nature of the relationship between the immune system and mood disorders. The data suggest potential differences in immune system and systemic inflammation markers in patients experiencing a depressive episode in MDD and BD, particularly in chemokines, PDGF-B, IL-9, sTNFR1, and IgG to oxidized LDL. Studying the role of these markers in the development of mood disorders may be crucial for understanding their pathophysiology and developing more targeted therapeutic strategies. Conversely, immune and inflammatory markers such as IL-1β, IL-4, IL-6, TNF-α, and CRP showed no significant differences between patients with MDD and BD. However, only a limited number of studies considered variables such as the subtype of BD and patient sex, which could impact the interpretation of findings. Further research is warranted to explore the associations between immune system parameters and mood disorders.