In English
Introduction
Anxiety, a term that resonates with discomfort and unease, is far more than a fleeting emotion. It represents a complex psychological state, often characterized by an amalgamation of tension, apprehensive thoughts, and physical changes such as elevated heart rate or blood pressure. Anxiety, in its clinical form, is not merely a transient response to stress but can evolve into a range of disorders, including generalized anxiety disorder, panic disorder, social anxiety disorder, and several others, that are among the most prevalent mental health challenges faced globally
[Javaid, 2023].
In 2019, the World Health Organization (WHO)
[Mental disorders [Electronic] estimated the worldwide prevalence of anxiety disorders as 4.4%, which amounted to approximately 301 million people at that time. The prevalence of anxiety disorders varies by region, age, sex, and over time. For instance, some studies suggested that anxiety disorders are more common in females than in males
[Burani, 2020] and that they can occur at any age, although adolescence or early adulthood is the most frequent period of the disorder onset
[Narmandakh, 2021]. According to a report from the Institute for Health Metrics and Evaluation (IHME)
[GBD 2019. Global, 2019], the number of people living with anxiety disorders globally may have increased over time due to population growth and aging, limited access to healthcare services, and epidemiological situations. In 2020, amid the COVID-19 pandemic, the number of people suffering from anxiety and depressive disorders increased by 26% and 28%, respectively, in one year alone
[Mc Carthy, 2024].
Mood and anxiety disorders are significant not only due to their prevalence but also because of the profound impact they have on individuals' lives. They can disrupt personal relationships, impair work performance, and erode the overall quality of life, making anxiety a matter of considerable clinical importance.
The spectrum of anxiety is broad, encompassing both acute and chronic manifestations
[Bados, 2010]. State anxiety represents the temporary experience of stress or nervousness in response to a specific situation perceived as threatening. It is a normal human reaction to stressors and typically resolves once the stressor is removed. On the other hand, trait anxiety refers to a more persistent and enduring tendency to experience anxiety across various situations. This aspect of anxiety is more akin to a personality characteristic, reflecting a stable predisposition to respond to anxiety even in the absence of immediate stressors. The distinction between state and trait anxiety is crucial for understanding the full scope of anxiety as a psychological phenomenon and for tailoring appropriate interventions.
The etiology of anxiety disorders is multifaceted, with genetic factors playing a significant role alongside environmental influences. Scientific research has long been involved in unravelling the genetic underpinnings of anxiety, with studies showing that both state and trait anxiety have heritable components
[Fox, 2021]. However, to date, there is no consensus on the degree of heritability, which ranges significantly between different types of studies. The twins study
[Kendler, 2008] estimated the heritability of anxiety disorders between 72 and 89%%, whereas the longitudinal study
[Smoller, 2020] produced a more conservative estimate of 25—30%%.
Despite the progress made in identifying genetic risk factors for anxiety disorders through twin and family studies, genome-wide association studies (GWAS)
[Levey, 2020], and candidate gene approaches
[Lindholm, 2020], much remains to be understood about the specific genetic variants involved and their mechanisms of action.
The rationale for the present study stems from the need to deepen our understanding of the genetic factors contributing to anxiety disorders. While previous research has laid the groundwork, there are still gaps in knowledge regarding how these genetic factors interact with environmental influences to precipitate and maintain both state and trait anxiety. Moreover, there is a need to explore whether genetic contributions differ between these two components of anxiety, which could have significant implications for prevention and treatment strategies.
This study aims to address these gaps by focusing on several research questions and objectives: First, we seek to estimate the association between variants in the BDNF and AMPD1 genes with state and trait anxiety. Second, we aim to elucidate how these genetic factors contribute to the biological pathways that underlie the development and persistence of anxiety. Third, we intend to compare the influence of genetics on state versus trait anxiety to determine if distinct genetic profiles underpin these different aspects of the condition.
By exploring these questions, our study hopes to contribute to the complex interplay between genetics and environmental factors in the etiology of anxiety disorders. This knowledge could lead to more personalized approaches to treatment, such as pharmacogenomics or targeted psychotherapeutic interventions. Additionally, it could inform preventive measures by identifying individuals at higher genetic risk for developing anxiety disorders. Ultimately, this research endeavors to improve outcomes for those suffering from anxiety disorders by laying the foundation for more effective and individualized care.
Trait and State Anxiety
Spielberger
et al.
[Spielberger, 1999] suggested that anxiety can be conceptualized in two ways: as a stable disposition and as a transient emotional state that everyone experiences from time to time by introducing the distinction between state anxiety and trait anxiety. Both trait anxiety and state anxiety were seen as unimodal constructs. State anxiety is defined as an unpleasant emotional response when coping with threatening or dangerous situations
[Pretorius, 2023], which includes a cognitive appraisal of the threat as a precursor to it occurring
[Smith, 1990]. In general, states refer to any characteristic that can be reliably measured, but "typically state variables refer to conscious, verbally reported qualities, such as mood"
[Corr, 2020]. Trait anxiety, on the other hand, refers to persistent individual differences in the tendency to respond with heightened state anxiety when anticipating a threatening situation. This tendency is present in a wide range of situations and is stable over time. Spielberger
[Saviola, 2020] defined trait anxiety as a general disposition to experience temporary anxious states and suggested that the two constructs were related.
However, it is still unclear whether these two types of anxiety are behaviorally connected or separate features. According to Spielberg's early theory, anxiety is a single-dimensional construct that includes both state and trait anxiety, viewed as two sides of the same coin. In this framework, an anxious individual has a personality trait coupled with a tendency for heightened episodic anxiety in dangerous or stressful situations. Nevertheless, some researchers proposed that trait and state anxiety are distinct multidimensional construct
[Barros, 2022].
Several studies
[Baur, 2013; Saviola, 2020; Leal, 2017] attempted to analyze the differences in psychological and physiological parameters associated with state and trait anxiety. Recent functional magnetic resonance imaging (fMRI) study
[Saviola, 2020] examined the neural basis of trait and state anxiety components by assessing the correlation between structural gray matter covariance and resting-state functional connectivity patterns with state and trait anxiety scores measured by the State-Trait Anxiety Inventory. The study provided evidence of neuroanatomical and functional distinctions between the two types of anxiety. It was shown that trait anxiety correlated with structural configurations, while state anxiety correlated with functional patterns of brain activity.
Similarly, Baur et al.
[Baur, 2013] used fMRI and diffusion tensor imaging to study the conjoint activity of the insula and amygdala and its association with state and trait anxiety. The study identified different psychological paths implicating two components of anxiety — while resting state functional connectivity was strongly associated with state anxiety, structural connectivity was positively correlated with trait anxiety.
Another study
[Leal, 2017] examined the relationship between state and trait anxiety, assuming a strong correlation between the two in the case of the unidimensional nature of anxiety and an absence of correlation if anxiety is a multidimensional construct. The study produced mixed evidence, showing a moderate positive correlation between state and trait anxiety in the situation when participants were subjected to an interpersonal threat. However, there was no correlation between two components of anxiety when participants were exposed to a physical threat (dental procedure).
Pathophysiology of anxiety
Mood and anxiety disorders are characterized by a variety of neuroendocrine, neurotransmitter, and neuroanatomical disruptions. Identifying the most functionally relevant differences is complicated by the high degree of interconnectivity between neurotransmitter- and neuropeptide-containing circuits in limbic, brain stem, and higher cortical brain areas
[Van Calker, 2019]. The conventional neurobiological hypothesis attributes noradrenergic, serotonergic, frontal lobe, and limbic systems as the most prominent biological pathways involved in anxiety. It has been suggested that reduced serotonin activity and elevated activity of the noradrenergic system are two main causal factors of the disorder onset
[Lai, 2023].
This study, however, explores a relatively new neurotrophic hypothesis, which associates the impairments in neuroplasticity implicating a deficiency of neurotrophic factors, such as brain-derived neurotrophic factor (BDNF), with the pathophysiology of anxiety
[Miranda, 2019]. Neuroplasticity refers to the ability of the nervous system to change its structure and function in response to experiences. This includes the growth of new neurons (neurogenesis), the formation of new synapses (synaptogenesis), and changes in synaptic strength (synaptic plasticity)
[Zelada, 2023]. These processes are essential for learning, memory, and the adaptation of the brain to new situations.
Neurotrophic factors are a family of proteins that support neurons' growth, survival, and differentiation. BDNF is one of the most extensively studied neurotrophic factors and is known to be crucial for neuroplasticity. It plays a significant role in regulating synaptic function and maintaining neuronal health
[Rauti, 2020]. According to the neurotrophic hypothesis of anxiety, reduced levels or activity of BDNF and possibly other neurotrophic factors can lead to decreased neuroplasticity, which in turn may contribute to the development of anxiety disorders. This could manifest as an impaired ability to adapt to stress, difficulty in extinguishing fear memories, or an increased vulnerability to environmental stressors.
Evidence supporting the neurotrophic hypothesis includes findings that individuals with anxiety disorders often have lower levels of BDNF in their blood compared to healthy controls
[Shafiee, 2024]. Furthermore, some treatments for anxiety, including antidepressants and physical exercise, have been shown to increase BDNF levels, which correlates with improvements in anxiety symptoms. Additionally, animal studies have shown that stress can reduce BDNF expression in the brain, particularly in regions associated with emotion regulation, such as the hippocampus and prefrontal cortex.
Our second hypothesis examined the contribution of the adenosine signaling system to anxiety. Adenosine is a naturally occurring nucleoside in the brain that functions as a central nervous system depressant. It modulates neuronal activity through its action on specific adenosine receptors, which are G protein-coupled receptors found throughout the brain
[Van Calker, 2019]. There are four known types of adenosine receptors: A1, A2A, A2B, and A3, each with different distributions and functions. Activation of adenosine A1 receptors generally has an inhibitory effect on neuronal activity, promoting sedation and anxiolytic (anxiety-reducing) effects. Conversely, activation of A2A receptors can have varying effects depending on their location in the brain but is often associated with wakefulness and potential anxiogenic (anxiety-producing) effects
[Van Calker, 2019].
Although the effect of adenosine receptors on anxiety disorder and depression has been commonly discussed in the research literature, this study focuses on the adenosine monophosphate deaminase (AMP deaminase), the enzyme that facilitates the conversion of inosine monophosphate to adenosine monophosphate, the precursor for adenosine. Therefore, AMP deaminase plays an important role in the regulation of the extracellular levels of adenosine in the brain, a molecule that acts as a neuromodulator and neuroprotectant in the central nervous system through purinergic receptors. By influencing adenosine levels, AMP deaminase indirectly participates in modulating neuronal excitability, neuroinflammation, and responses to stress.
Methods
Participant recruitment and selection criteria
This study included 73 individuals of Caucasian descent. All participants were healthy adults aged 25 to 45, residing in the federal territory of Sirius (the Russian Federation). Participants had diverse baseline characteristics and volunteered to take part in the research project. To ensure the research's safety and transparency, participants signed an informed consent form approved by the ethics committee of Sirius University of Science and Technology. Data collection and management were carried out in accordance with the research protocols, guaranteeing the confidentiality of participants' personal data and adhering to the principles of fairness, transparency, and ethical conduct in the research.
Trait and state anxiety scoring
Trait and state anxiety levels were assessed using Spielberger's state-trait anxiety inventory (STAI) with the adaptation of the Russian language by Y.L. Khanin
[Karelin, 2005; Spielberger, 1983]. The STAI is the most widely used instrument to assess anxiety levels in healthy and clinical participants due to its reliability and psychometric validity
[Gustafson, 2020].
The Spielberger self-completed anxiety questionnaire consists of 40 questions that assess an individual's level of state and trait anxiety. The questions in the survey were rated on a four-point scale. Participants were asked to indicate the intensity of their feelings at the moment, ranging from "not at all" to "very much" for state anxiety questions. For trait anxiety questions, participants were asked to indicate the frequency of such states ranging from "rarely" to "almost always."
Raw scores were reversed, and total test scores were calculated, ranging from 20 to 80 points, where a higher score corresponds to a higher anxiety level. Based on the severity of symptoms, participants are classified into one of three groups: low anxiety (up to 30 points), medium anxiety (31 to 44 points), and high anxiety (45 points and above) for each anxiety component. Since anxiety is a condition that can be measured on a continuous scale based on the severity of symptoms, our study focuses on the extreme end of this scale, which represents a pathological level of anxiety. We have compared two groups of participants in our analysis: one group with low to medium anxiety scores ranging from 0 to 44 and another group with high anxiety scores of 45 or more, which places them in the top rank of the scale.
DNA isolation
Buccal swab samples were collected from all participants using a sterile, disposable medical cotton swab. Each participant was instructed not to eat, drink, smoke, or chew gum for at least 30 minutes prior to sample collection. The swabs were rubbed against the inner surface of the participant's cheek for about 30 seconds. The swab was then placed into the tube containing a buffer solution (PBS, 0.5 M Hepes and 0.1 M EDTA) and stored at a temperature of +4C.
DNA was extracted and purified using the physical method with spin columns with silicate sorbent diaGene (Dia-M, Moscow, Russia, article number 3489.0250) following the manufacturer's protocol. The quantity of extracted DNA was assessed using a NanoDrop spectrophotometer (Thermo Fisher Scientific) and the real-time PCR. PCR kit with fluorescent probes Biomaster HS-qPCR (Biolabmix, Russia), a buffer, a set of highly specific primers, and probes for amplification was used to detect polymorphisms in the AMPD1and BDNF genes.
Genetic association analysis
Target genes for gene association analysis were selected based on previous research findings and their biological relevance to anxiety disorders. We focused on two genes encoding the risk factors of interest: the
BDNF gene regulating transportation and secretion of the BDNF protein
[Arévalo, 2023] and the
AMPD1 gene encoding AMP deaminase, the enzyme involved in the synthesis of adenosine
[Van Calker, 2019]. While the choice of the
BDNF gene is well supported by the previous research in the field of affective disorders, the inclusion of the
AMPD1 gene expands the conventional area of investigation by shifting the focus of research from the genes regulating adenosine receptors to the gene involved in adenosine metabolism. Although
AMPD1 is highly expressed in skeletal muscles and studied in the context of energy metabolism, cellular function, and metabolic disorders, new evidence suggests its potential relevance to mental health conditions and psychiatric phenotypes
[Zhang, 2015].
We used instrumental variable analysis, a statistical method used to infer causality in observational studies. The instrumental variable (IV) is a variable associated with the exposure of interest (in this case, BDNF protein and AMP deaminase levels) but is not associated with the outcome (anxiety) except through its effect on the exposure.
The
BDNF and
AMPD1 genes, which encode the BDNF protein and AMP deaminase enzyme, can be used as instrumental variables in this context. Genetic variants, or single nucleotide polymorphisms (SNPs), in these genes, can affect the levels of BDNF protein and adenosine produced in the body. These SNPs can be used as an IV because they are randomly assigned
[Sanderson, 2022] at conception (Mendelian randomization) and thus are not affected by confounding factors that might influence both BDNF protein or adenosine levels and anxiety.
In this study, we used the BDNF and AMPD1 genes as IV; more specifically, by reviewing previous genetic studies, we identified SNPs in both genes that are associated with BDNF protein (rs6265) and adenosine levels (rs17602729).
Next, the associations of these SNPs with anxiety were assessed by comparing the prevalence of pathological levels of anxiety in individuals with different genotypes at these SNPs. The association of the index SNPs with anxiety suggests that BDNF protein and adenosine may play a causal role in anxiety.
Statistical methods
Data were analyzed using R statistical software. Anxiety scores were presented as the mean ± standard deviation. The scores were assessed using Kolmogorov-Smirnov’s test for distribution normality and Bartlet’s test for homoscedasticity. No impediments to the use of parametric tests were found for any of the evaluated parameters. A level of significance of 5% was considered. No obstacles were found for any of the evaluated parameters when performing parametric tests, with a the significance level of 5% considered.
The correlation between state and trait anxiety was assessed with Pearson’s correlation test. The correlation coefficient was interpreted in accordance with a conventional standard - low (r < 0.50), moderate (0,50 ≤ r ≤ 0,75), and high (r > 0.75)
Generalized linear regression models adjusted by sex and age were used to estimate the effect of the minor alleles on the anxiety levels. Point estimates and p-values were reported in the result section.
Hardy—Weinberg equilibrium was assessed by the chi-square test, and the genotype and allele frequencies were compared between the participants with low/medium rank and high rank of anxiety. Linkage disequilibrium between paired SNPs was analyzed, and the degree of linkage disequilibrium between SNPs was expressed as D′. The value of D′ ranges from 0 to 1, with a higher value indicating a higher degree of linkage disequilibrium between the two loci.
Results
Baseline characteristics
The study population is composed of 73 individuals with an average age of 34,9 years (±8,8). The population is almost equally divided by sex, with 36 males (49,3%) and 37 females (50,7%). In terms of trait anxiety levels, a significant majority of the population, 59 individuals or 82%, have low to medium levels of trait anxiety. The remaining 18% or 14 individuals have high levels of trait anxiety.
The average state anxiety score for the entire population is 35,0 (±10,1). However, there is a noticeable difference when divided by trait anxiety levels: those with low/medium trait anxiety have an average state anxiety score of 31,1 (±6,6), while those with high trait anxiety have a significantly higher average state anxiety score of 51,3 (±3,9). Baseline characteristics stratified by the trait anxiety status (low/ medium vs. high) are shown in Table 1.
Table 1
Baseline characteristics
|
|
Trait anxiety
(low/medium level)
59 (82%)
|
Trait anxiety
(high level)
14 (18%)
|
Total
73 (100%)
|
|
Age (yr.)
|
34,9 (±9,2)
|
34,7 (±7,3)
|
34,9 (±8,8)
|
|
Sex (M)
|
30 (50,8%)
|
6 (42,9%)
|
36 (49,3%)
|
|
Sex (F)
|
29 (49,2%)
|
8 (57,1%)
|
37 (50,7%)
|
|
State anxiety
|
31,1 (±6,6)
|
51,3 (±3,9)
|
35,0 (±10,1)
|
|
Trait anxiety
|
35,3 (±6,8)
|
49,5 (±10,4)
|
38,0 (±9,4)
|
Note. The values of continuous variables are shown in M (±SD), representing mean and standard deviation, respectively. The values of categorical variables are shown as SUM (%), representing sum of the values and percentage from the total, respectively.
Correlation analysis
A strong positive correlation existed between state and trait anxiety, and Pearson’s correlation coefficient was R2 = 0,72 (95% CI 0,58, 0,81). Table 2 shows both outcomes' mean, SD, and correlation coefficients.
Table 2
Means, standard deviations, and correlations with confidence intervals between state and trait anxiety scores
|
Outcome
|
M
|
SD
|
R2
|
|
State anxiety score
|
35,01
|
10,15
|
|
|
Trait anxiety score
|
38,03
|
9,43
|
0,72**
|
|
|
|
|
[0,58, 0,81]
|
Note. M and SD are used to represent mean and standard deviation, respectively. Values in square brackets indicate the 95% confidence interval for each correlation. The confidence interval is a plausible range of population correlations that could have caused the sample correlation (Cumming, 2014). * indicates p < 0,05. ** indicates p < 0,01.
Genetic association analysis
The index SNPs for the analysis were chosen in accordance with the existing knowledge of their associations with biomarkers of interest. Several checks were employed to ensure directional concordance between the genotype data of the Sirius residents and the European populations' genotype data. Frequencies of the major alleles in the European population, as reported by Ensembl and observed in the current project, were compared. The difference between frequencies is within 3%, which indicates that the frequencies in all three populations are similar (Table 3).
Table 3
The comparison of minor allele frequencies in the Sirius population and Ensemble
|
RSID
|
Minor allele Sirius
|
Minor allele Ensembl
|
Minor allele freqency Sirius
|
Minor allele freqency Ensembl
|
Freqency difference between Sirius and Ensembl
|
|
rs6265
|
T
|
T
|
0,47
|
0,5
|
-0,03
|
|
rs17602729
|
A
|
A
|
0,14
|
0,14
|
0
|
Genotype frequency analysis found no significant association of the AMPD1 genotype with trait anxiety, but for state anxiety, χ 2 analysis showed a significant association (Table 4). The frequency of minor allele heterozygotes in low/medium vs. high state anxiety subjects was 75,9% vs. 57,1% and 28,6% vs. 33,3% for low/medium vs. high trait anxiety subjects, respectively. The minor allele in rs17602729 appeared to be associated with a higher level of state anxiety after adjusting for age and sex using a logistic regression model.
Similarly, genotype frequency analysis of the BDNF genotype was not associated with state anxiety but showed a statistically significant association with trait anxiety (Table 4). The frequency of minor allele homozygotes in low/medium vs. high state anxiety subjects was 13,8% vs. 21,4% and 8,9% vs. 33,3% for low/medium vs. high trait anxiety subjects, respectively. The minor allele in rs6265 tends to increase the level of trait anxiety after adjusting for age and sex using a logistic regression model.
Table 4
Contingency table analysis of AMPD1 and BDNF genotype frequencies in subjects with low/medium anxiety levels compared with those who have high anxiety levels
|
Group
|
Cases (n)
|
AMPD1
|
|
rs17602729
|
|
GG
|
GA
|
AA
|
χ2
|
P-value
|
|
State Anxiety
|
|
|
|
|
|
|
|
Low/medium score
|
58 (100%)
|
14 (25%)
|
44 (75%)
|
0 (0%)
|
4,34
|
0,04*
|
|
High score
|
14 (100%)
|
6 (43%)
|
8 (57%)
|
0 (0%)
|
|
|
|
Sum
|
72 (100%)
|
20 (28%)
|
52 (72%)
|
0 (0%)
|
|
|
|
Trait Anxiety
|
|
|
|
|
|
|
|
Low/medium score
|
56 (100%)
|
41 (73%)
|
16 (27%)
|
0 (0%)
|
0,01
|
0,93
|
|
High score
|
15 (100%)
|
10 (67%)
|
5 (33%)
|
0 (0%)
|
|
|
|
Sum
|
71 (100%)
|
51 (72%)
|
21 (28%)
|
0 (0%)
|
|
|
| |
|
BDNF
|
| |
|
rs6265
|
| |
|
CC
|
CT
|
TT
|
χ2
|
P-value
|
|
State Anxiety
|
|
|
|
|
|
|
|
Low/medium score
|
58 (100%)
|
12 (21%)
|
38 (65%)
|
8 (14%)
|
0,56
|
0,76
|
|
High score
|
14 (100%)
|
3 (21.5%)
|
8 (57%)
|
3 (21.5%)
|
|
|
|
Sum
|
72 (100%)
|
15 (21%)
|
46 (64%)
|
11 (15%)
|
|
|
|
Trait Anxiety
|
|
|
|
|
|
|
|
Low/medium score
|
56 (100%)
|
15 (27%)
|
36 (64%)
|
5 (9%)
|
8,01
|
0,02*
|
|
High score
|
15 (100%)
|
0 (0%)
|
10 (67%)
|
5 (33%)
|
|
|
|
Sum
|
71 (100%)
|
15 (21%)
|
46 (65%)
|
10 (14%)
|
|
|
| |
|
|
|
|
|
|
|
Note. Statistically significant P-values are marked with *
|
|
|
|
|
Fig. 1 shows the distribution of the trait anxiety scores by the BDNF genotype coded as a dominant model. Participants with at least one copy of the minor allele have an average trait anxiety score higher than those who do not have minor alleles. A concordant association is shown for the AMPD1 genotype. Participants with at least one copy of the minor allele have a mean state anxiety score higher than those who have homozygous major alleles.
The general linear regression model estimated that minor alleles in rs17602729 were associated with a 0,3 (p-value 0,031) point higher state anxiety score. The directionally concordant effect of rs6265 on the trait anxiety was 0,4 (p-value 0,011) points higher in those with minor alleles.
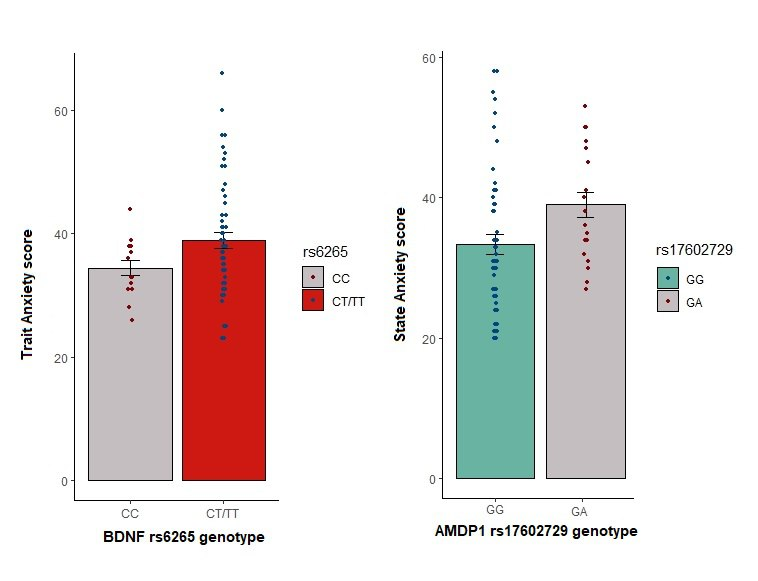
Fig. 1. Trait anxiety scores by the BDNF genotype and state anxiety scores by the AMPD1 genotype are both coded as a dominant model.
Discussion
This study aimed to investigate the association between the BDNF and AMPD1 genes with trait and state anxiety levels. The analysis showed that the BDNF gene was associated with trait anxiety; the presence of the minor allele in the individual genotype increased the level of trait anxiety by 0,4 points. The AMPD1 gene was associated with state anxiety, and a copy of the minor allele was associated with 0,3 higher state anxiety.
The association of the
BDNF gene with trait anxiety provides additional evidence supporting the hypothesis that lower BDNF expression may be associated with higher anxiety levels. BDNF is a neurotrophin that plays a crucial role in brain plasticity and neuronal survival. Previous studies have indicated that BDNF is involved in the pathophysiology of various psychiatric disorders, including anxiety disorders
[Lin, 2020]. Our findings align with this body of research, suggesting that lower BDNF levels may indeed contribute to increased anxiety symptoms.
One of the primary observations from our study was the inverse relationship between BDNF expression and anxiety levels. Participants with genetically determined lower BDNF levels exhibited higher scores on anxiety measurement scales. This trend suggests that BDNF may play a protective role against anxiety, and its deficiency could potentially lead to heightened anxiety levels.
The findings of our study reveal a significant association between genetically determined lower adenosine levels and increased anxiety levels. This aligns with previous research suggesting that adenosine, a neuromodulator with inhibitory effects in the central nervous system, plays a crucial role in modulating anxiety behavior.
The association of the AMPD1 gene with state anxiety supports the adenosine hypothesis. Adenosine is known to mediate several physiological processes, including sleep, arousal, and stress response. Our results indicate that a deficiency in adenosine may disrupt these processes, leading to heightened responses to the stressors. This could be due to an imbalance in neural excitability and inhibition, which has been implicated in the pathophysiology of anxiety disorders.
Interestingly, our findings also suggest that the effect of adenosine on anxiety levels may be dose-dependent, with genetically determined lower levels of adenosine associated with higher anxiety levels, while moderate to high levels appeared to have an anxiolytic effect
[Van Calker, 2019]. This is consistent with the dual role of adenosine in the central nervous system, where it can act both as a neuroprotectant and a neurotoxin, depending on its concentration.
However, while our results are promising, it is important to note that they do not establish a causative relationship between BDNF and AMPD1 expression and anxiety. The observed association could be influenced by various other factors not accounted for in this study. For instance, environmental stressors, interactions, or other neurochemical imbalances could also play a role in modulating anxiety levels.
Moreover, our research did not delve into the specific mechanisms through which
BDNF and
AMPD1 might influence anxiety. Previous research has suggested that BDNF might impact anxiety through its effects on brain structures such as the hippocampus and amygdala, which are crucially involved in stress response and emotion regulation
[Baur, 2013]. In contrast, adenosine impacts anxiety through several potential biological pathways, primarily through its interaction with adenosine receptors in the brain
[Van Calker, 2019]. Future research should aim to elucidate these underlying mechanisms further.
Conclusion
In conclusion, our findings suggested a significant association between the BDNF and AMPD1 genes and anxiety. Those genes are implicated in different components of anxiety; while BDNF is associated with trait anxiety, a more stable over-time individual characteristic, AMPD1, appeared to influence the extent of the response to a stressor. Although these two components of anxiety are correlated, the underlying biological mechanisms differ.
